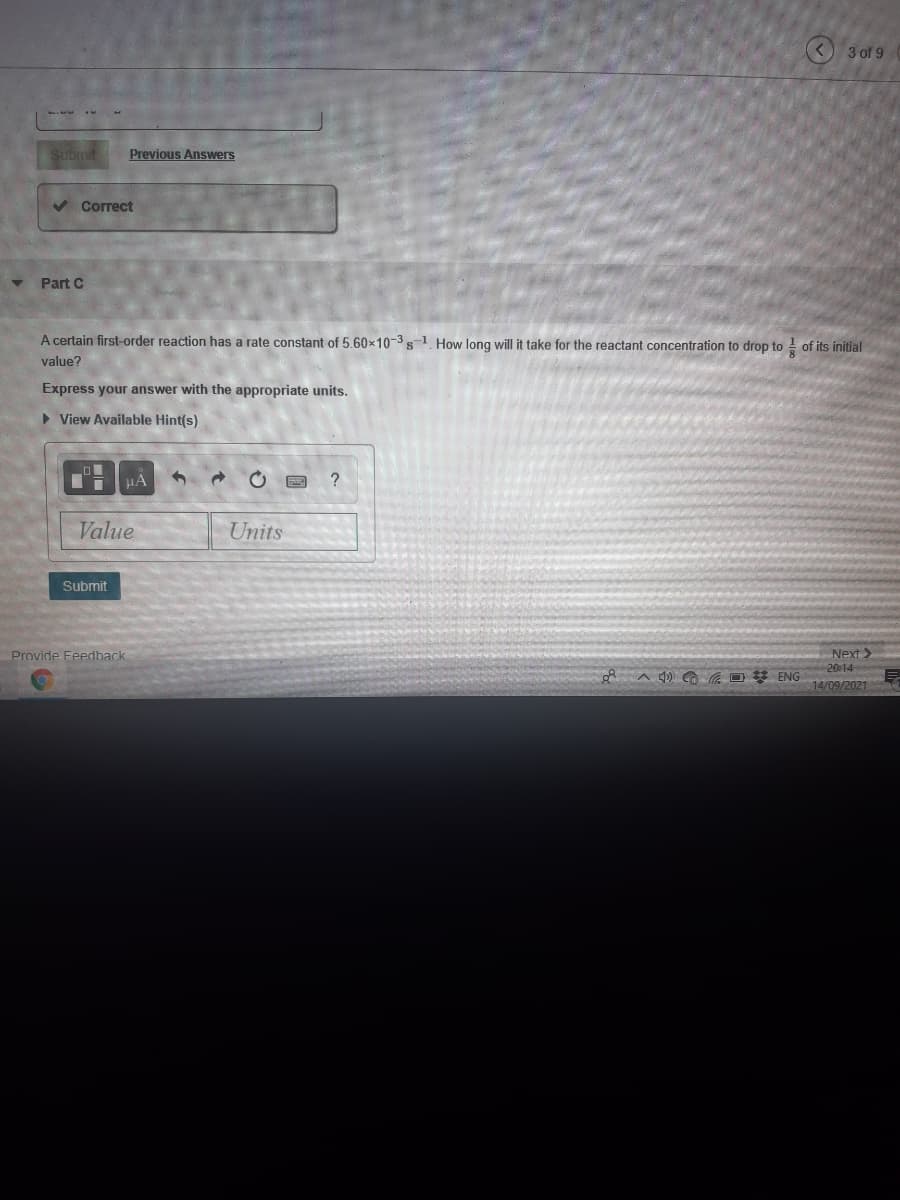
A certain first-order reaction has a rate constant of 5.60x10-3s 1 How long will it take for the reactant concentration to drop to of its initial value? Express your answer with the appropriate units. View Available Hint(s) HA Value Units Submit
A certain first-order reaction has a rate constant of 5.60x10-3s 1 How long will it take for the reactant concentration to drop to of its initial value? Express your answer with the appropriate units. View Available Hint(s) HA Value Units Submit
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter11: Rate Of Reaction
Section: Chapter Questions
Problem 98QAP
Related questions
Question
Part C please
Transcribed Image Text:3 of 9
Submit
Previous Answers
V Correct
Part C
A certain first-order reaction has a rate constant of 5.60x10-3 s1 How long will it take for the reactant concentration to drop to of its initial
value?
Express your answer with the appropriate units.
• View Available Hint(s)
HA
?
Value
Units
Submit
Provide Feedback
Next>
2014
) ☺ O ENG
14/09/2021
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
