For your temperature studies in Part Ba student obtain the results below for the temperatures and rate constants. Calculate the activation energy in units of kJ/mol. Data Table Temperature (°C) Rate constant (k, appropriate units based on reaction order) 17 6,689,537,124 41.8 18,563,418,549 2.1 1,247,348,345 Note: The easiest way to do this is by creating a plot in Excel, as you will do in lab. These numbers are made up, and will be different from those you determine in lab. Report your answer with one place after the decimal.
For your temperature studies in Part Ba student obtain the results below for the temperatures and rate constants. Calculate the activation energy in units of kJ/mol. Data Table Temperature (°C) Rate constant (k, appropriate units based on reaction order) 17 6,689,537,124 41.8 18,563,418,549 2.1 1,247,348,345 Note: The easiest way to do this is by creating a plot in Excel, as you will do in lab. These numbers are made up, and will be different from those you determine in lab. Report your answer with one place after the decimal.
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter20: Kinetics
Section: Chapter Questions
Problem 20.64E: Recently, researchers studying the kinetics of metal atom reactions with small gas molecules...
Related questions
Question
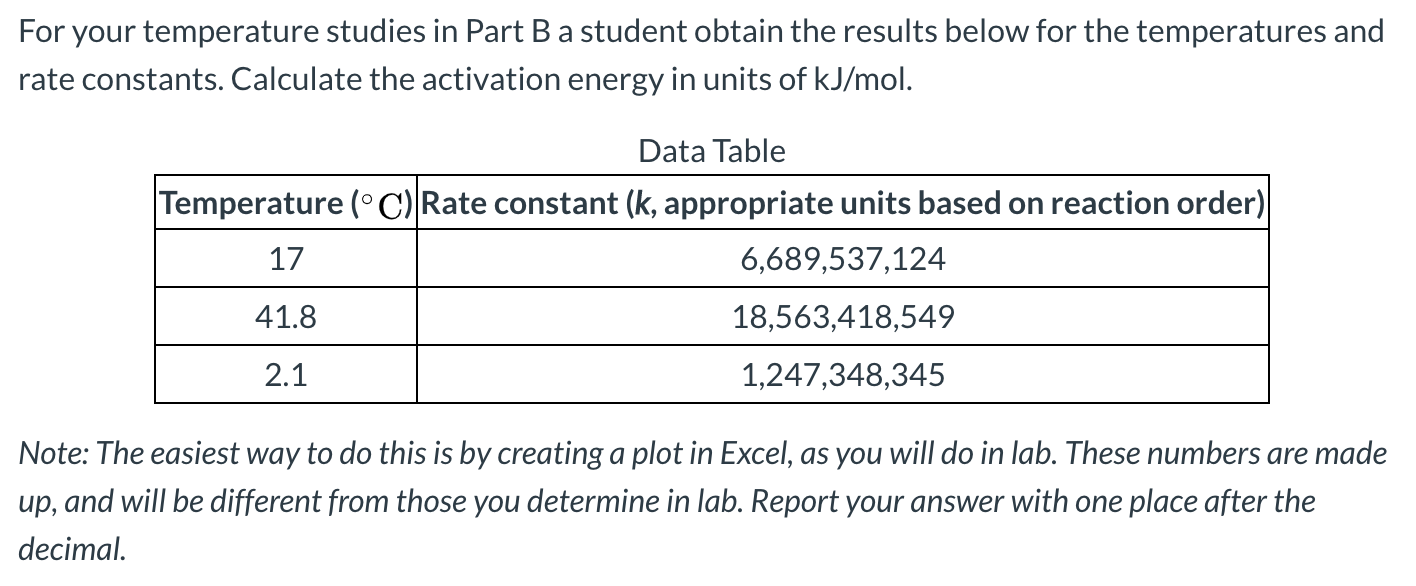
Transcribed Image Text:For your temperature studies in Part Ba student obtain the results below for the temperatures and
rate constants. Calculate the activation energy in units of kJ/mol.
Data Table
Temperature (°C) Rate constant (k, appropriate units based on reaction order)
17
6,689,537,124
41.8
18,563,418,549
2.1
1,247,348,345
Note: The easiest way to do this is by creating a plot in Excel, as you will do in lab. These numbers are made
up, and will be different from those you determine in lab. Report your answer with one place after the
decimal.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole