A certain half-reaction has a standard reduction potential Eed = +0.01 V. An engineer proposes using this half-reaction at the cathode of a galvanic cell that must provide at least 1.00 V of electrical power. The cell will operate under standard conditions. Note for advanced students: assume the engineer requires this half-reaction to happen at the cathode of the cell. olo ローロ Is there a minimum standard reduction potential that the half-reaction used at the anode of this cell can have? O yes, there is a minimum. = Uv red If so, check the "yes" box and calculate the minimum. Round your answer to 2 decimal places. If there is no lower limit, check the "no" box. O no minimum Is there a maximum standard reduction potential that the half-reaction used at the anode of this cell can have? yes, there is a maximum. Ov red If so, check the "yes" box and calculate the maximum. Round your answer to 2 decimal places. If there is no upper limit, check the "no" box. no maximum By using the information in the ALEKS Data tab, write a balanced equation describing a half reaction that could be used at the anode of this cell. Note: write the half reaction as it would actually occur at the anode.
A certain half-reaction has a standard reduction potential Eed = +0.01 V. An engineer proposes using this half-reaction at the cathode of a galvanic cell that must provide at least 1.00 V of electrical power. The cell will operate under standard conditions. Note for advanced students: assume the engineer requires this half-reaction to happen at the cathode of the cell. olo ローロ Is there a minimum standard reduction potential that the half-reaction used at the anode of this cell can have? O yes, there is a minimum. = Uv red If so, check the "yes" box and calculate the minimum. Round your answer to 2 decimal places. If there is no lower limit, check the "no" box. O no minimum Is there a maximum standard reduction potential that the half-reaction used at the anode of this cell can have? yes, there is a maximum. Ov red If so, check the "yes" box and calculate the maximum. Round your answer to 2 decimal places. If there is no upper limit, check the "no" box. no maximum By using the information in the ALEKS Data tab, write a balanced equation describing a half reaction that could be used at the anode of this cell. Note: write the half reaction as it would actually occur at the anode.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 60P
Related questions
Question
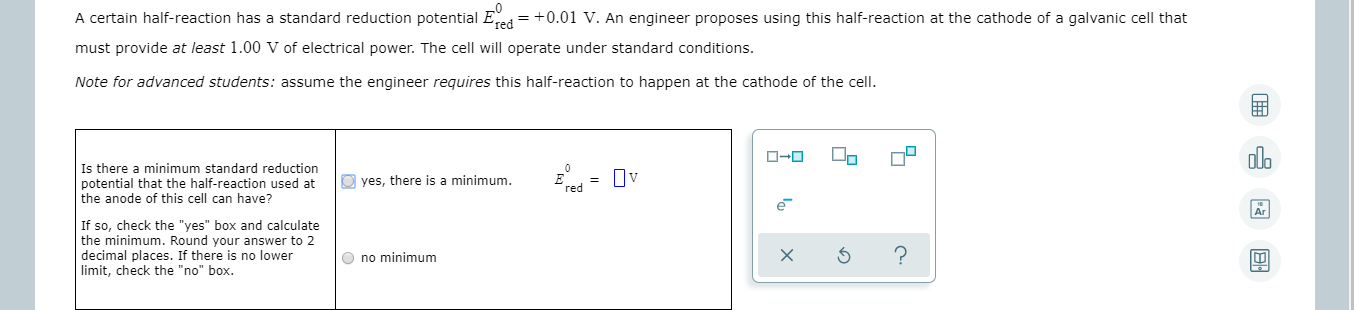
Transcribed Image Text:A certain half-reaction has a standard reduction potential Eed = +0.01 V. An engineer proposes using this half-reaction at the cathode of a galvanic cell that
must provide at least 1.00 V of electrical power. The cell will operate under standard conditions.
Note for advanced students: assume the engineer requires this half-reaction to happen at the cathode of the cell.
olo
ローロ
Is there a minimum standard reduction
potential that the half-reaction used at
the anode of this cell can have?
O yes, there is a minimum.
= Uv
red
If so, check the "yes" box and calculate
the minimum. Round your answer to 2
decimal places. If there is no lower
limit, check the "no" box.
O no minimum
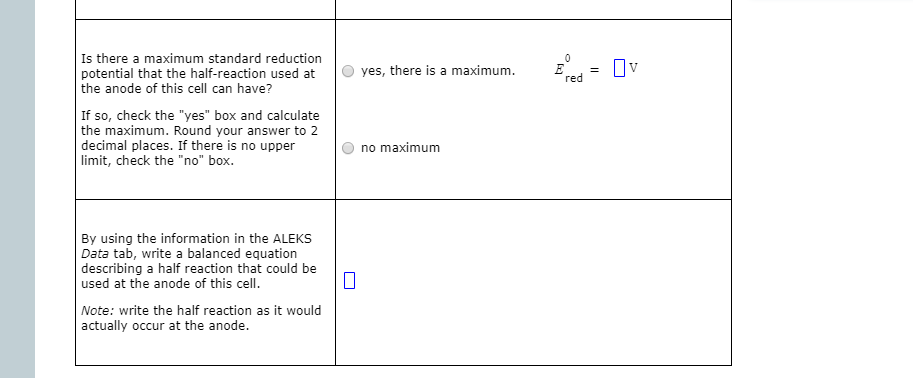
Transcribed Image Text:Is there a maximum standard reduction
potential that the half-reaction used at
the anode of this cell can have?
yes, there is a maximum.
Ov
red
If so, check the "yes" box and calculate
the maximum. Round your answer to 2
decimal places. If there is no upper
limit, check the "no" box.
no maximum
By using the information in the ALEKS
Data tab, write a balanced equation
describing a half reaction that could be
used at the anode of this cell.
Note: write the half reaction as it would
actually occur at the anode.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax
