A certain liquid x has a normal freezing point of -8.10 °c and a freezing point depression constant K₁=2.69 °C kg mol. A solution is prepared by dissolving some glycine (C₂H,NO₂) in 600. g of x. This solution freezes at -10.4 °c. Calculate the mass of C₂H₂NO₂ that was dissolved. Round your answer to 2 significant digits. g x10
A certain liquid x has a normal freezing point of -8.10 °c and a freezing point depression constant K₁=2.69 °C kg mol. A solution is prepared by dissolving some glycine (C₂H,NO₂) in 600. g of x. This solution freezes at -10.4 °c. Calculate the mass of C₂H₂NO₂ that was dissolved. Round your answer to 2 significant digits. g x10
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter7: Equilibria In Multiple-component Systems
Section: Chapter Questions
Problem 7.55E: Determine how ideal the following solutions are by calculating the mole fraction of solute in each...
Related questions
Question
Kf kb eq..
Transcribed Image Text:< >
|||
Watch Online Foundat...
AA
O STATES OF MATTER
Using the Kf and Kb equations with...
g
Explanation
www-awa.aleks.com
C clumperrucksey.life/ln... X
Check
ALEKS- Andrew Herr...
1/5
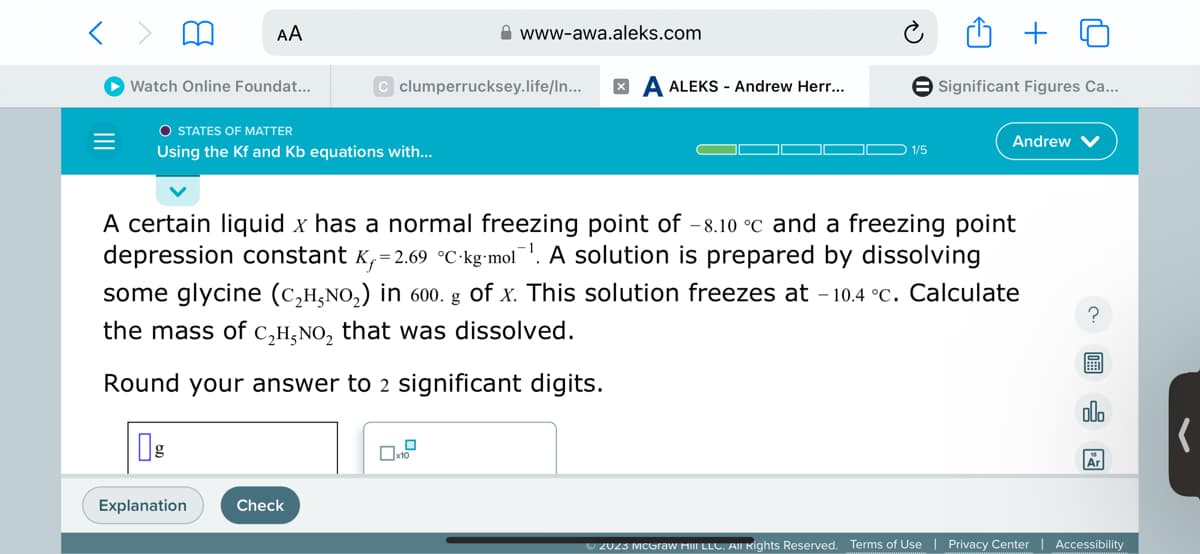
A certain liquid x has a normal freezing point of -8.10 °c and a freezing point
depression constant K₁=2.69 °C-kg-mol¹. A solution is prepared by dissolving
some glycine (C₂H,NO₂) in 600. g of x. This solution freezes at -10.4 °C. Calculate
the mass of C₂H₂NO₂ that was dissolved.
Round your answer to 2 significant digits.
Significant Figures Ca...
Andrew
?
allo
Ar
© 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accessibility
(
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,


Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,
