A chemical engineer is studying the following reaction: 2 NO(g)+2H2(g) → N,(9)+2H,0(g) At the temperature the engineer picks, the equilibrium constant K, for this reaction is 0.17. The engineer charges ("fills") four reaction vessels with nitrogen monoxide and hydrogen, and lets the reaction begin. She then measures the composition of the mixture inside each vessel from time to time. Her first set of measurements are shown in the table below. Predict the changes in the compositions the engineer should expect next time she measures the compositions. reaction vessel compound pressure expected change in pressure NO 3.80 atm f increase CI decrease (no change) H2 5.43 atm f increase I decrease (no change) А N2 7.72 atm f increase I decrease (no change) H,0 3.07 atm f increase C+ decrease (no change) NO 4.19 atm f increase I decrease (no change) H, 5.64 atm f increase I decrease (no change) В N2 6.22 atm f increase I decrease (no change) H,0 1.83 atm f increase I decrease (no change) NO 3.39 atm f increase I decrease (no change) H2 4.84 atm f increase I decrease (no change) N2 6.62 atm f increase I decrease (no change) H,0 2.63 atm C T increase CI decrease O (no change) B.
A chemical engineer is studying the following reaction: 2 NO(g)+2H2(g) → N,(9)+2H,0(g) At the temperature the engineer picks, the equilibrium constant K, for this reaction is 0.17. The engineer charges ("fills") four reaction vessels with nitrogen monoxide and hydrogen, and lets the reaction begin. She then measures the composition of the mixture inside each vessel from time to time. Her first set of measurements are shown in the table below. Predict the changes in the compositions the engineer should expect next time she measures the compositions. reaction vessel compound pressure expected change in pressure NO 3.80 atm f increase CI decrease (no change) H2 5.43 atm f increase I decrease (no change) А N2 7.72 atm f increase I decrease (no change) H,0 3.07 atm f increase C+ decrease (no change) NO 4.19 atm f increase I decrease (no change) H, 5.64 atm f increase I decrease (no change) В N2 6.22 atm f increase I decrease (no change) H,0 1.83 atm f increase I decrease (no change) NO 3.39 atm f increase I decrease (no change) H2 4.84 atm f increase I decrease (no change) N2 6.62 atm f increase I decrease (no change) H,0 2.63 atm C T increase CI decrease O (no change) B.
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter12: Chemical Equilibrium
Section: Chapter Questions
Problem 12.41PAE: Because calcium carbonate is a sink for CO32- in a lake, the student in Exercise 12.39 decides to go...
Related questions
Question
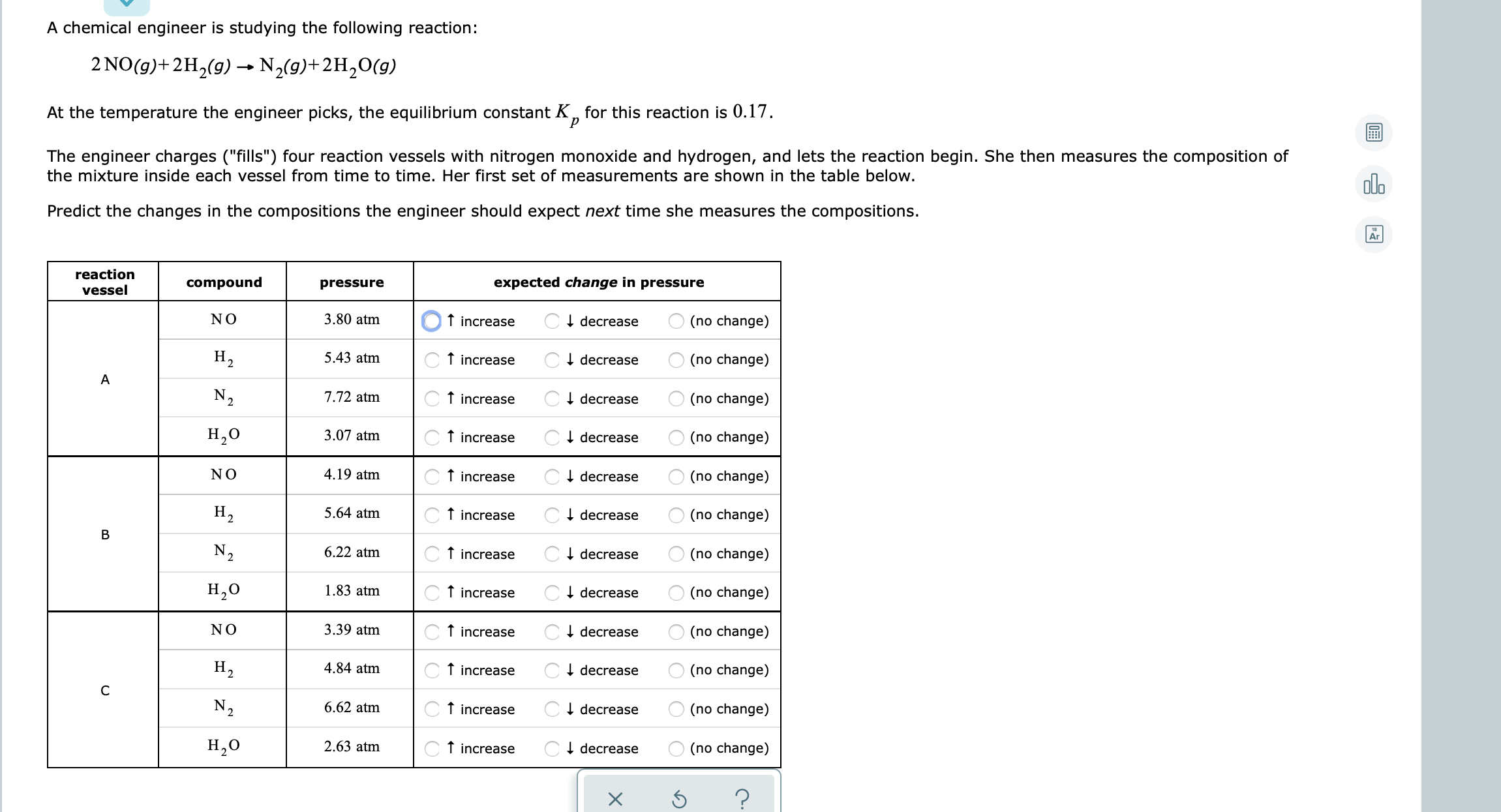
Transcribed Image Text:A chemical engineer is studying the following reaction:
2 NO(g)+2H2(g) → N,(9)+2H,0(g)
At the temperature the engineer picks, the equilibrium constant K, for this reaction is 0.17.
The engineer charges ("fills") four reaction vessels with nitrogen monoxide and hydrogen, and lets the reaction begin. She then measures the composition of
the mixture inside each vessel from time to time. Her first set of measurements are shown in the table below.
Predict the changes in the compositions the engineer should expect next time she measures the compositions.
reaction
vessel
compound
pressure
expected change in pressure
NO
3.80 atm
f increase
CI decrease
(no change)
H2
5.43 atm
f increase
I decrease
(no change)
А
N2
7.72 atm
f increase
I decrease
(no change)
H,0
3.07 atm
f increase
C+ decrease
(no change)
NO
4.19 atm
f increase
I decrease
(no change)
H,
5.64 atm
f increase
I decrease
(no change)
В
N2
6.22 atm
f increase
I decrease
(no change)
H,0
1.83 atm
f increase
I decrease
(no change)
NO
3.39 atm
f increase
I decrease
(no change)
H2
4.84 atm
f increase
I decrease
(no change)
N2
6.62 atm
f increase
I decrease
(no change)
H,0
2.63 atm
C T increase
CI decrease
O (no change)
B.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning