A chemical engineer is studying the following reaction: HCN(aq)+ NH,(aq) CN (aq)+NH,(aq) At the temperature the engineer picks, the equilibrium constant K, for this reaction is 0.83, The engineer charges ("fills") four reaction vessels with hydrogen cyanide and ammonia, and lets the reaction begin. He then measures the composition of the mixture inside each vessel from time to time. His first set of measurements are shown in the table below. Predict the changes in the compositions the engineer should expect next time he measures the compositions. reaction vessel compound concentration expected change in concentration HCN 0.48 M O T increase Ol decrease O (no change) NH, 0.40 M O f increase OI decrease O (no change) CN 0.89 M O t increase OI decrease O (no change) NH, 0 98 M O t increase OI decrease O (no change) HCN 106 M O increase O decrease O (no change) NH, 0 95 M O fimerease OI decrease O (no change) CN 085 M O increase Ou decrease O (no change) NH, 0 98 M Aincrease OI decrease O (no change) HCN 037 M 0 ncruase decrease O (no change) NH, 0 29 M Oerease 0. decrease O (no change) CN 100 M O t inrease derease NH, 1 09 M decreaE e dange) Explanation Check
A chemical engineer is studying the following reaction: HCN(aq)+ NH,(aq) CN (aq)+NH,(aq) At the temperature the engineer picks, the equilibrium constant K, for this reaction is 0.83, The engineer charges ("fills") four reaction vessels with hydrogen cyanide and ammonia, and lets the reaction begin. He then measures the composition of the mixture inside each vessel from time to time. His first set of measurements are shown in the table below. Predict the changes in the compositions the engineer should expect next time he measures the compositions. reaction vessel compound concentration expected change in concentration HCN 0.48 M O T increase Ol decrease O (no change) NH, 0.40 M O f increase OI decrease O (no change) CN 0.89 M O t increase OI decrease O (no change) NH, 0 98 M O t increase OI decrease O (no change) HCN 106 M O increase O decrease O (no change) NH, 0 95 M O fimerease OI decrease O (no change) CN 085 M O increase Ou decrease O (no change) NH, 0 98 M Aincrease OI decrease O (no change) HCN 037 M 0 ncruase decrease O (no change) NH, 0 29 M Oerease 0. decrease O (no change) CN 100 M O t inrease derease NH, 1 09 M decreaE e dange) Explanation Check
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter12: Chemical Equilibrium
Section: Chapter Questions
Problem 12.41PAE: Because calcium carbonate is a sink for CO32- in a lake, the student in Exercise 12.39 decides to go...
Related questions
Question
Can you please explain step by step where you find the answer.
Thank you!
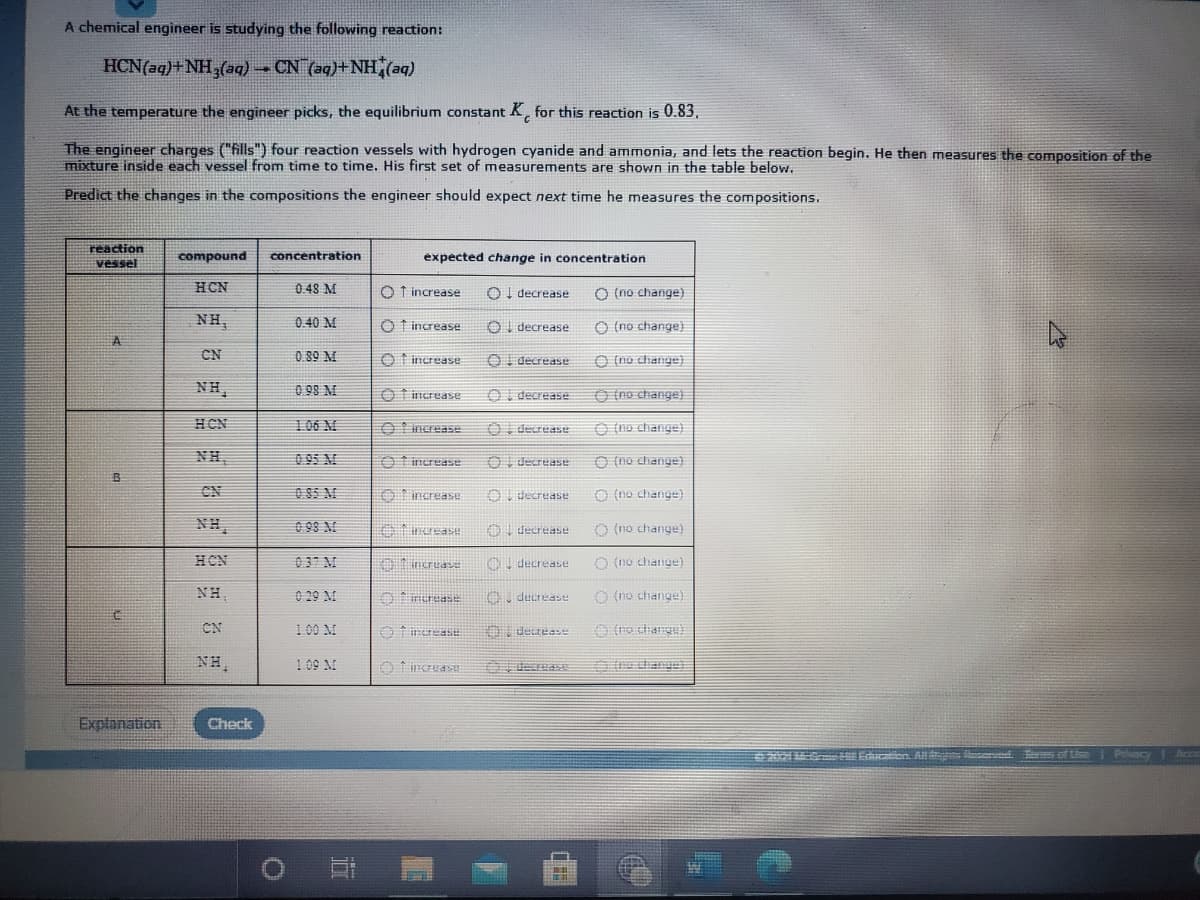
Transcribed Image Text:A chemical engineer is studying the following reaction:
HCN(aq)+NH,(aq) CN (aq)+NH,(aq)
At the temperature the engineer picks, the equilibrium constant K, for this reaction is 0.83,
The engineer charges ("fills") four reaction vessels with hydrogen cyanide and ammonia, and lets the reaction begin. He then measures the composition of the
mixture inside each vessel from time to time. His first set of measurements are shown in the table below.
Predict the changes in the compositions the engineer should expect next time he measures the compositions.
reaction
vessel
compound
concentration
expected change in concentration
HCN
0.48 M
O f increase
Ol decrease
O (no change)
NH,
0.40 M
O f increase
OI decrease
O (no change)
CN
0.89 M
O t increase
OI decrease
O (no change)
NH,
0 98 M
O t increase
OI decrease
O (no change)
HCN
106 M
O Iincrease
O decrease
O (no change}
NH,
0 95 M
O f increase
OI decrease
O (no change)
CN
O 85 M
Oincrease
OI decrease
O (no change)
NH,
0 98 M
Aincrease
O decrease
O (no change)
HCN
037 M
01 ncruase
decrease
O (no change)
NH,
0 29 M
Orerease
O decrease
O (no change)
CN
100 M
O decrease
NH,
109 M
O fincras
decreas
dhange)
Explanation
Check
20MG H Edaten All end s of tise i P yI Ace
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning