A chemist is attempting to synthesize, or create ammonia, NH3 in the laboratory. He reacts 14.6g of nitrogen, N, with hydrogen gas, H, to form 27.9g of ammonia, NH3. Determine the mass of hydrogen that was used in the reaction.
A chemist is attempting to synthesize, or create ammonia, NH3 in the laboratory. He reacts 14.6g of nitrogen, N, with hydrogen gas, H, to form 27.9g of ammonia, NH3. Determine the mass of hydrogen that was used in the reaction.
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter5: Atomic Theory : The Nuclear Model Of The Atom
Section: Chapter Questions
Problem 62E
Related questions
Question
Please answer these questions

Transcribed Image Text:8) A chemistry student reacts a black metallic element with an element that exists as a
bright blue powder. What will the resulting chemical compound look like?
A) The compound will appear mostly black since the color black will overpower the
bright blue color
B) The appearance of the chemical compound is not dependent on the color of the
original elements.
C) The color of the resulting compound will be a mixture of black and bright blue,
leaning mainly towards the color black.
D) The color of the resulting compound will be a mixture of black and bright blue,
leaning mainly towards the color bright blue.
9) A 98.4g sample of sulfuric acid, H2SO., was found to contain 32.4g of sulfur, S. What is
the percent by mass of sulfur in the sample?
10) In a round-bottom flask, a chemist completely reacts 4g of hydrogen with 25g of
chlorine. What is the percent by mass of hydrogen in the compound that was formed?
11) Two unknown compounds are tested to determine their composition. Compound A was
found to contain, 16g of magnesium and 130g of sulfur. Compound B contains 9g of
magnesium and 63g of sulfur. Are these the same compound? Explain your answer
12) A chemical analysis of two unknown compounds found that they are both made up of
only calcium and oxygen. During an analysis of their mass, it was found that they both
contain the same percent by mass of calcium. However, the instrument used in the
analysis broke before the mass of oxygen could be determined. With only the
information that is known, can you be sure that both compounds are the same?
Transcribed Image Text:ment.pdf
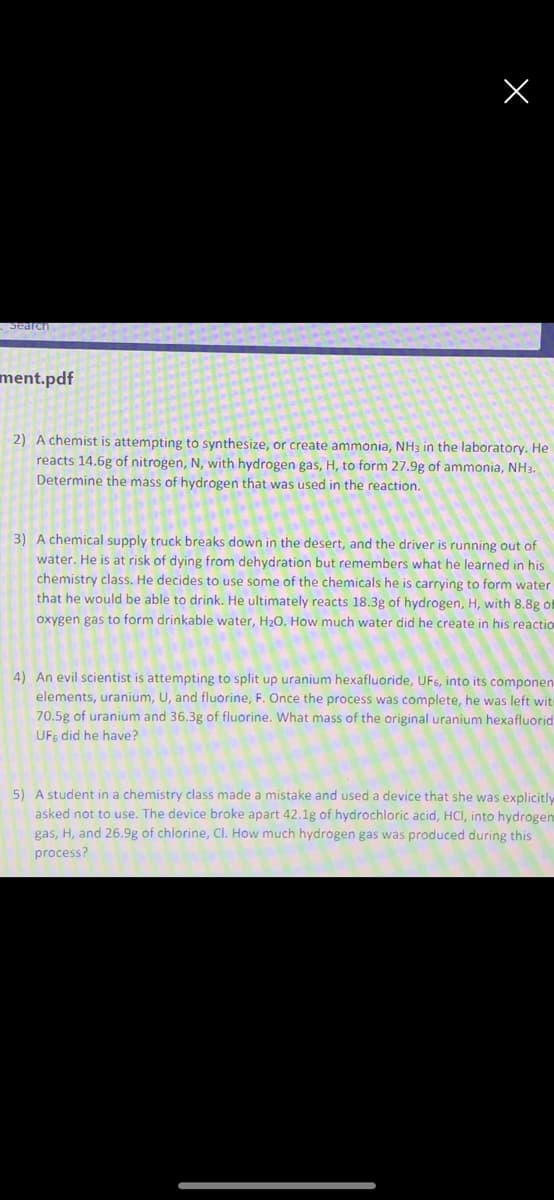
2) A chemist is attempting to synthesize, or create ammonia, NH3 in the laboratory. He
reacts 14.6g of nitrogen, N, with hydrogen gas, H, to form 27.9g of ammonia, NH3.
Determine the mass of hydrogen that was used in the reaction.
3) A chemical supply truck breaks down in the desert, and the driver is running out of
water. He is at risk of dying from dehydration but remembers what he learned in his
chemistry class. He decides to use some of the chemicals he is carrying to form water
that he would be able to drink. He ultimately reacts 18.3g of hydrogen, H, with 8.8g of
oxygen gas to form drinkable water, H20. How much water did he create in his reactio
4) An evil scientist is attempting to split up uranium hexafluoride, UF6, into its componen.
elements, uranium, U, and fluorine, F. Once the process was complete, he was left witi
70.5g of uranium and 36.3g of fluorine. What mass of the original uranium hexafluorid
UFs did he have?
5) A student in a chemistry class made a mistake and used a device that she was explicitly
asked not to use. The device broke apart 42.1g of hydrochloric acid, HCI, into hydrogen
gas, H, and 26.9g of chlorine, Cl. How much hydrogen gas was produced during this
process?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning