A chemist measures the energy change AH during the following reaction: C₂Hg(g)+50₂(g) → 3 CO₂(g) + 4H₂O(l) Use the information to answer the following questions. This reaction is... Suppose 71.3 g of C3Hg react. Will any heat be released or absorbed? If you said heat will be released or absorbed in the second part of this question, calculate how much heat will be released or absorbed. Round your answer to 3 significant digits. AH=-2220. kJ endothermic. O exothermic. O Yes, absorbed. O Yes, released. O No. 0kJ 0 x10 X
A chemist measures the energy change AH during the following reaction: C₂Hg(g)+50₂(g) → 3 CO₂(g) + 4H₂O(l) Use the information to answer the following questions. This reaction is... Suppose 71.3 g of C3Hg react. Will any heat be released or absorbed? If you said heat will be released or absorbed in the second part of this question, calculate how much heat will be released or absorbed. Round your answer to 3 significant digits. AH=-2220. kJ endothermic. O exothermic. O Yes, absorbed. O Yes, released. O No. 0kJ 0 x10 X
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter6: Thermochemistry
Section: Chapter Questions
Problem 49E: The overall reaction in a commercial heat pack can be represented as 4Fe(s)+3O2(g)2Fe2O3(s)H=1652KJ...
Related questions
Question
100%
last question 3sig figs
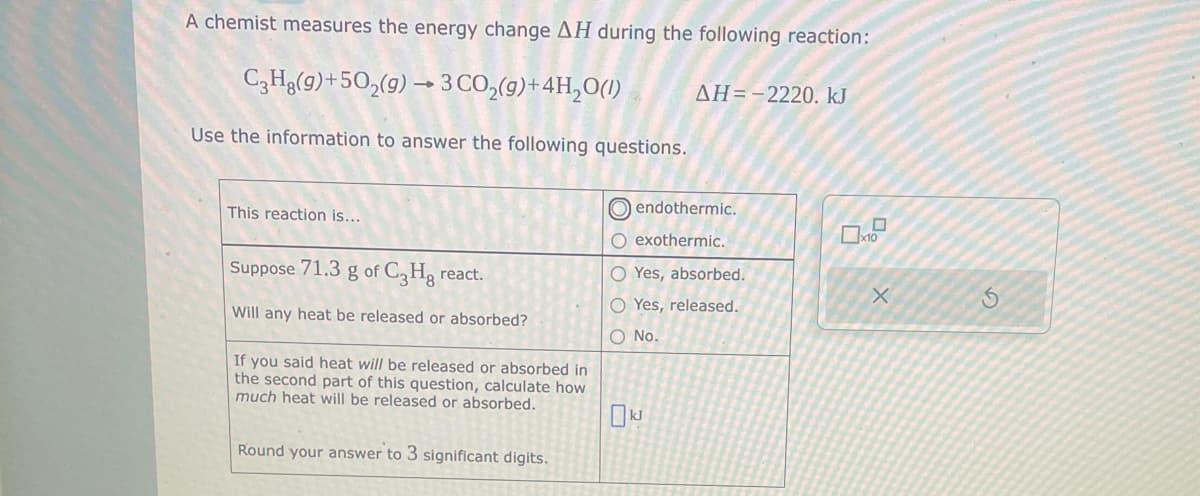
Transcribed Image Text:A chemist measures the energy change AH during the following reaction:
C3H8(g)+50₂(g) → 3 CO₂(g) + 4H₂O(1)
Use the information to answer the following questions.
This reaction is...
Suppose 71.3 g of C3Hg react.
Will any heat be released or absorbed?
If you said heat will be released or absorbed in
the second part of this question, calculate how
much heat will be released or absorbed.
Round your answer to 3 significant digits.
AH=-2220. kJ
endothermic.
O exothermic.
O Yes, absorbed.
O Yes, released.
O No.
kJ
0
x10
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning