A chemist must prepare 500. mL of 95.0 mM aqueous potassium permanganate (KMNO,) working solution. She'll do this by pouring out some 0.332 mol L aqueous potassium permanganate stock solution into a graduated cylinder and diluting it with distilled water. Calculate the volume in mL of the potassium permanganate stock solution that the chemist should pour out. Round your answer to 3 significant digits.
A chemist must prepare 500. mL of 95.0 mM aqueous potassium permanganate (KMNO,) working solution. She'll do this by pouring out some 0.332 mol L aqueous potassium permanganate stock solution into a graduated cylinder and diluting it with distilled water. Calculate the volume in mL of the potassium permanganate stock solution that the chemist should pour out. Round your answer to 3 significant digits.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter1: Matter And Measurements
Section: Chapter Questions
Problem 54QAP: Potassium sulfate has a solubility of 15 g/ 100 g water at 40C. A solution is prepared by adding...
Related questions
Question
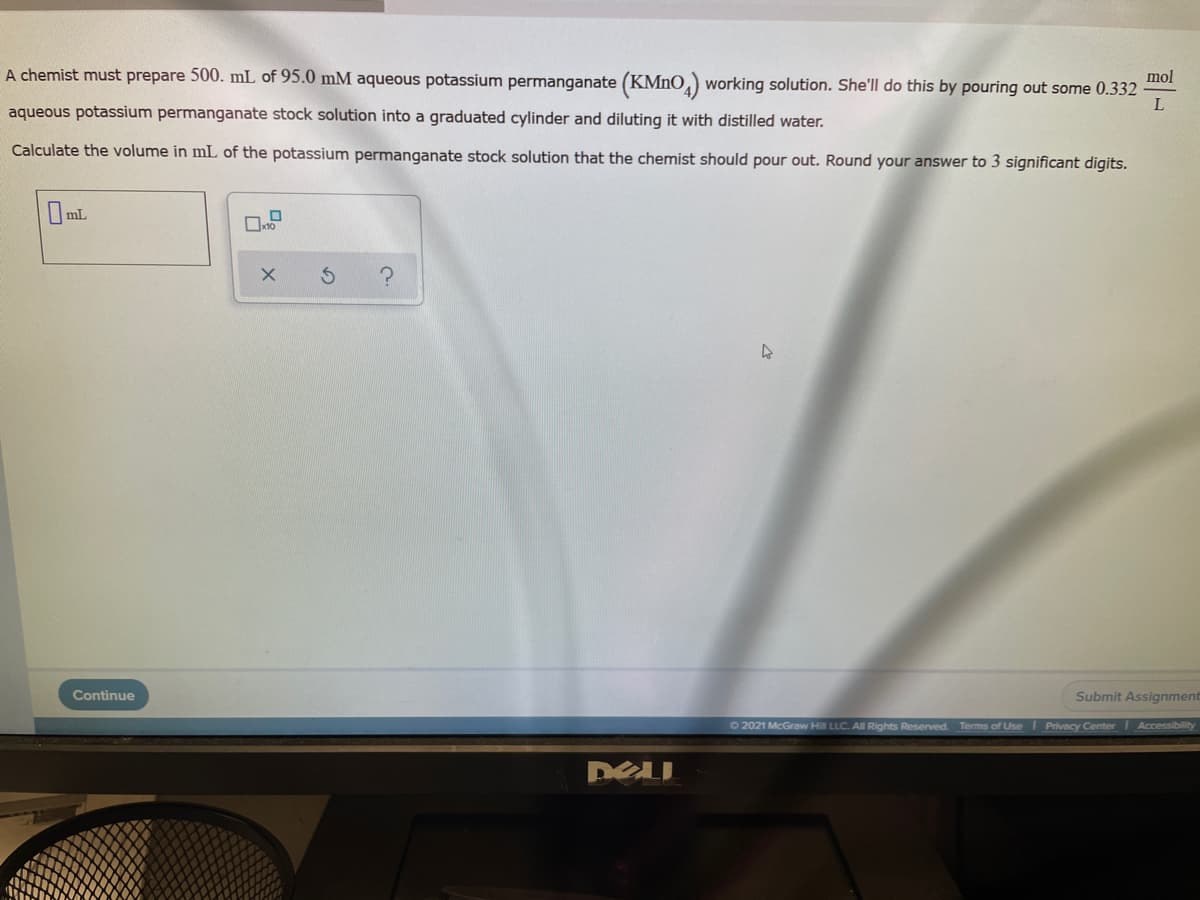
Transcribed Image Text:A chemist must prepare 500. mL of 95.0 mM aqueous potassium permanganate (KMNO, working solution. She'll do this by pouring out some 0.332
mol
L
aqueous potassium permanganate stock solution into a graduated cylinder and diluting it with distilled water.
Calculate the volume in mL of the potassium permanganate stock solution that the chemist should pour out. Round your answer to 3 significant digits.
Continue
Submit Assignment
O2021 McGraw H LLC. AlI Rights Reserved.
Terms of Use I Privacy Center Accessibility
DELI
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning