A chemist prepares a solution of sodium carbonate (Na₂CO,) by weighing out 50.7 mg of sodium carbonate into a 250. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in g/L of the chemist's sodium carbonate solution. Be sure your answer has the correct number of significant digits. 0²/ 0.P X
A chemist prepares a solution of sodium carbonate (Na₂CO,) by weighing out 50.7 mg of sodium carbonate into a 250. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in g/L of the chemist's sodium carbonate solution. Be sure your answer has the correct number of significant digits. 0²/ 0.P X
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter1: Introduction To Chemistry
Section: Chapter Questions
Problem 1.89PAE: 1.89 Imagine that you place a cork measuring 1.30cm4.50cm3.00cm in a pan of water. On top of this...
Related questions
Question
Subject:
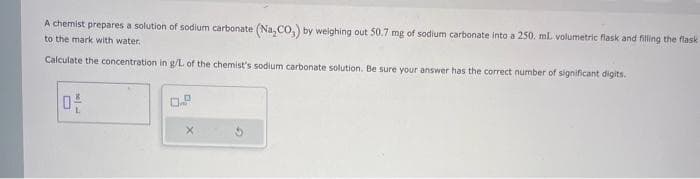
Transcribed Image Text:A chemist prepares a solution of sodium carbonate (Na₂CO,) by weighing out 50.7 mg of sodium carbonate into a 250. mL volumetric flask and filling the flask
to the mark with water.
Calculate the concentration in g/L of the chemist's sodium carbonate solution. Be sure your answer has the correct number of significant digits.
70
0.P
X
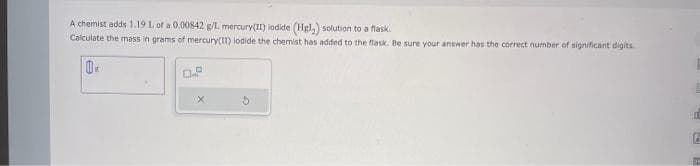
Transcribed Image Text:A chemist adds 1.19 1. of a 0.00842 g/L mercury(II) iodide (Hgl,) solution to a flask.
Calculate the mass in grams of mercury(11) lodide the chemist has added to the flask. Be sure your answer has the correct number of significant digits
0€
0
D
X
L
a
6
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning