Select the energy diagram or diagrams that illustrate reactions in which the second step is rate determining. A Free Energy Free Energy [Reaction A Reaction B Reaction C Reaction Coordinate Reaction D D Free IMM Energy B Reaction Coordinate Free Energy Reaction Energy Diagrams Reaction Coordinate Reaction Coordinate
Select the energy diagram or diagrams that illustrate reactions in which the second step is rate determining. A Free Energy Free Energy [Reaction A Reaction B Reaction C Reaction Coordinate Reaction D D Free IMM Energy B Reaction Coordinate Free Energy Reaction Energy Diagrams Reaction Coordinate Reaction Coordinate
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter18: Chemical Kinetics
Section: Chapter Questions
Problem 24P
Related questions
Question
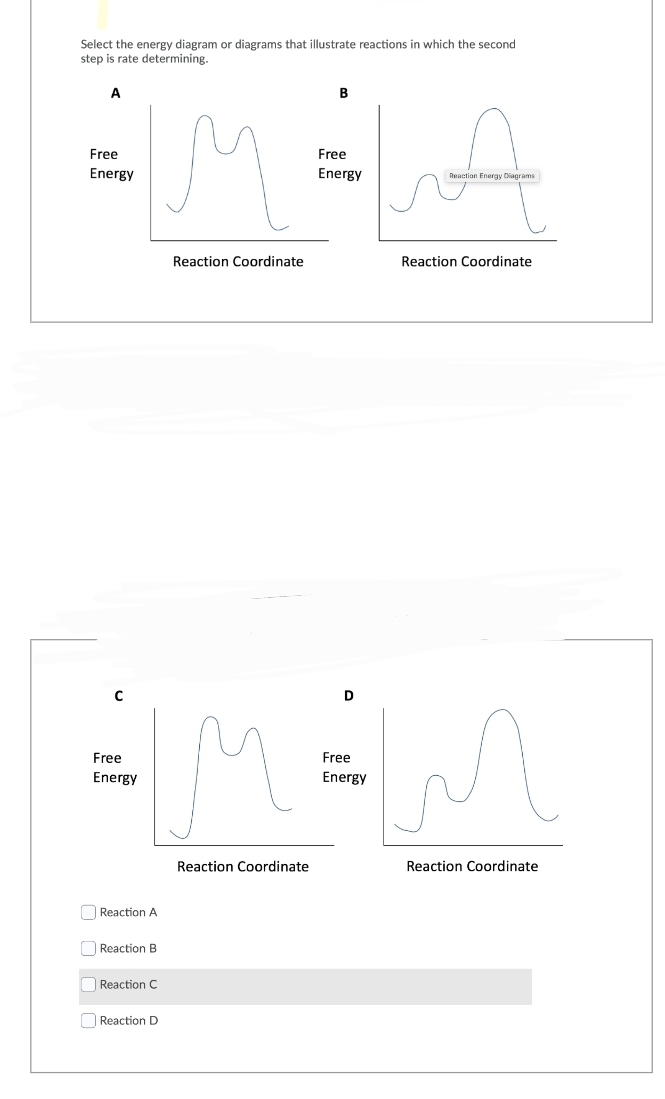
Transcribed Image Text:Select the energy diagram or diagrams that illustrate reactions in which the second
step is rate determining.
A
Free
Energy
C
Free
Energy
Reaction A
Reaction B
Reaction C
Reaction D
Reaction Coordinate
B
Reaction Coordinate
Free
Energy
Reaction Energy Diagrams
Reaction Coordinate
D
Free
M=MM
Energy
Reaction Coordinate

Transcribed Image Text:Select all of the following that would occur to an enzyme-catalyzed reaction in
the presence of a reversible, uncompetitive enzyme inhibitor (compared to the
same reaction in the absence of the inhibitor).
Vmax would increase
Km would increase
Vmax would decrease
Vmax would not change
Km would decrease
Km would not change
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co