A chemist wants to perform a reaction using the reagent below, which can act as a Bronsted-Lowry base. Considering only the reagent's capacity to act as a base, check the boxes of any compounds in the table that the chemist should not use as the solvent for the reaction. Use the ALEKS Data resource to look up useful data. If all of the solvents below are acceptable, please check the box that says "All solvents are acceptable." All solvents are acceptable reagent = ONa proposed solvent i OH 요.. OH do not use this solvent (check all that apply) D
A chemist wants to perform a reaction using the reagent below, which can act as a Bronsted-Lowry base. Considering only the reagent's capacity to act as a base, check the boxes of any compounds in the table that the chemist should not use as the solvent for the reaction. Use the ALEKS Data resource to look up useful data. If all of the solvents below are acceptable, please check the box that says "All solvents are acceptable." All solvents are acceptable reagent = ONa proposed solvent i OH 요.. OH do not use this solvent (check all that apply) D
Chapter30: Resolution Of (6)-a-phenylethylamine And Determination Of Optical Purity
Section: Chapter Questions
Problem 1Q
Related questions
Question
100%
Please answer 1 & 2. Thanks!
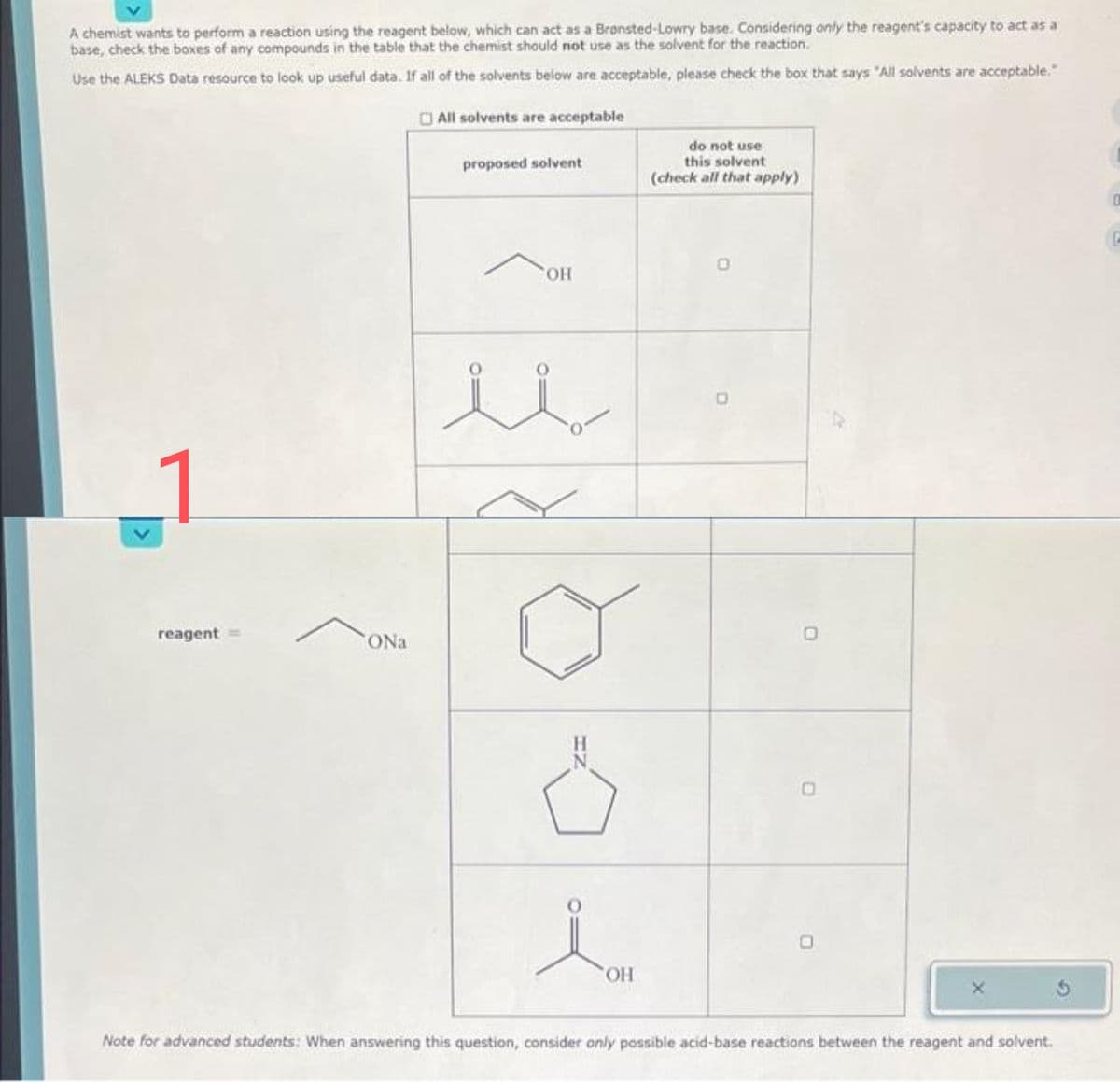
Transcribed Image Text:A chemist wants to perform a reaction using the reagent below, which can act as a Bransted-Lowry base. Considering only the reagent's capacity to act as a
base, check the boxes of any compounds in the table that the chemist should not use as the solvent for the reaction.
Use the ALEKS Data resource to look up useful data. If all of the solvents below are acceptable, please check the box that says "All solvents are acceptable."
All solvents are acceptable
reagent
ONa
proposed solvent
OH
Ö
OH
do not use
this solvent
(check all that apply)
O
Note for advanced students: When answering this question, consider only possible acid-base reactions between the reagent and solvent.
0
C
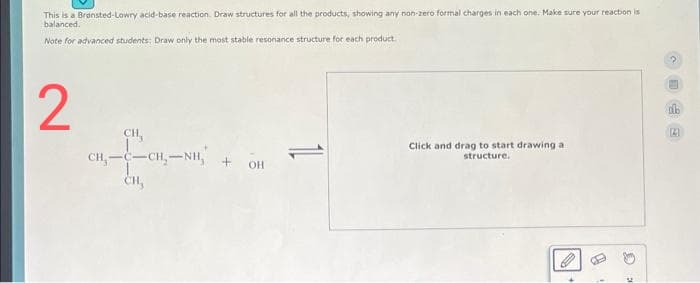
Transcribed Image Text:This is a Brønsted-Lowry acid-base reaction. Draw structures for all the products, showing any non-zero formal charges in each one. Make sure your reaction is
balanced.
Note for advanced students: Draw only the most stable resonance structure for each product.
2
CH,
CH,——CH,NH,
CH₂
+OH
Click and drag to start drawing a
structure.
C
SE
db
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT