A chemist wishes to convert iron into iron(II) bromide using this reaction: Fe(s) + 2 HBr(aq). → FeBr2(aq) + H2(g) She mixes small pieces of iron with 0.2 M aqueous HBr at room temperature and discovers that the reaction proceeds slowly. Select two ways she could speed up the reaction. cool the reaction mixture heat the reaction mixture increase the HBr concentration decrease the HBr concentration
A chemist wishes to convert iron into iron(II) bromide using this reaction: Fe(s) + 2 HBr(aq). → FeBr2(aq) + H2(g) She mixes small pieces of iron with 0.2 M aqueous HBr at room temperature and discovers that the reaction proceeds slowly. Select two ways she could speed up the reaction. cool the reaction mixture heat the reaction mixture increase the HBr concentration decrease the HBr concentration
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter17: Equilibrium
Section: Chapter Questions
Problem 3QAP: How do chemists envision reactions taking place in terms of the collision model for reactions? Give...
Related questions
Question
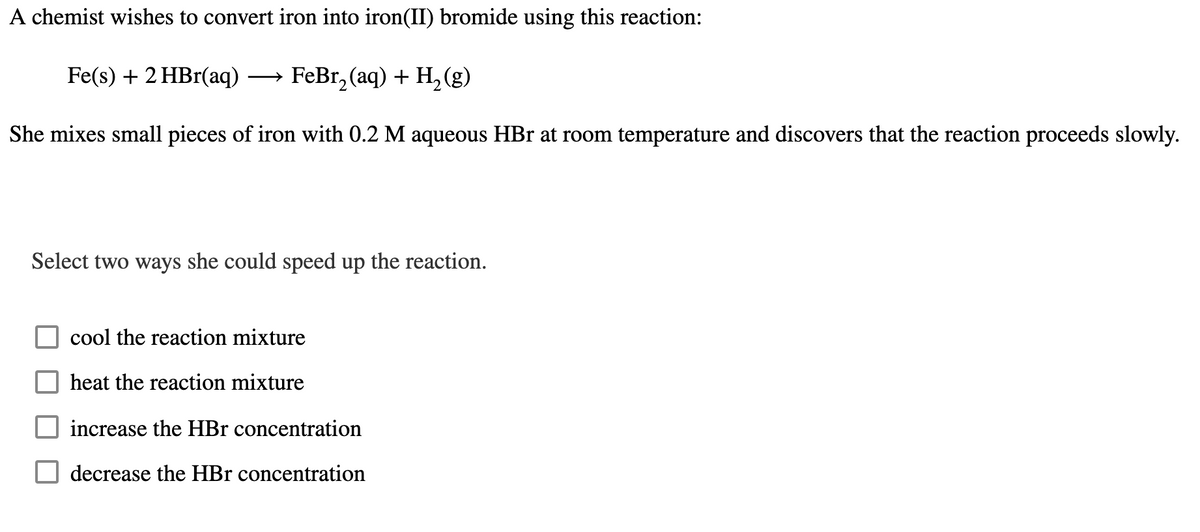
Transcribed Image Text:A chemist wishes to convert iron into iron(II) bromide using this reaction:
Fe(s) + 2 HBr(aq). → FeBr2(aq) + H2(g)
She mixes small pieces of iron with 0.2 M aqueous HBr at room temperature and discovers that the reaction proceeds slowly.
Select two ways she could speed up the reaction.
cool the reaction mixture
heat the reaction mixture
increase the HBr concentration
decrease the HBr concentration
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning