A chemistry graduate student is given 100. mL of a 1.80M trimethylamine ((CH3),N) solution. Trimethylamine is a weak base with K,=7.4 × 10". What mass of (CH,, NHC1 should the student dissolve in the (CH3),N solution to turn it into a buffer with pH =10.99? 3/3 You may assume that the volume of the solution doesn't change when the (CH;) NHC1 is dissolved in it. Be sure your answer has a unit symbol, and round it /3 to 2 significant digits.
A chemistry graduate student is given 100. mL of a 1.80M trimethylamine ((CH3),N) solution. Trimethylamine is a weak base with K,=7.4 × 10". What mass of (CH,, NHC1 should the student dissolve in the (CH3),N solution to turn it into a buffer with pH =10.99? 3/3 You may assume that the volume of the solution doesn't change when the (CH;) NHC1 is dissolved in it. Be sure your answer has a unit symbol, and round it /3 to 2 significant digits.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter15: Additional Aqueous Equilibria
Section: Chapter Questions
Problem 93QRT: When 40.00 mL of a weak monoprotic acid solution is titrated with 0.100-M NaOH, the equivalence...
Related questions
Question
100%
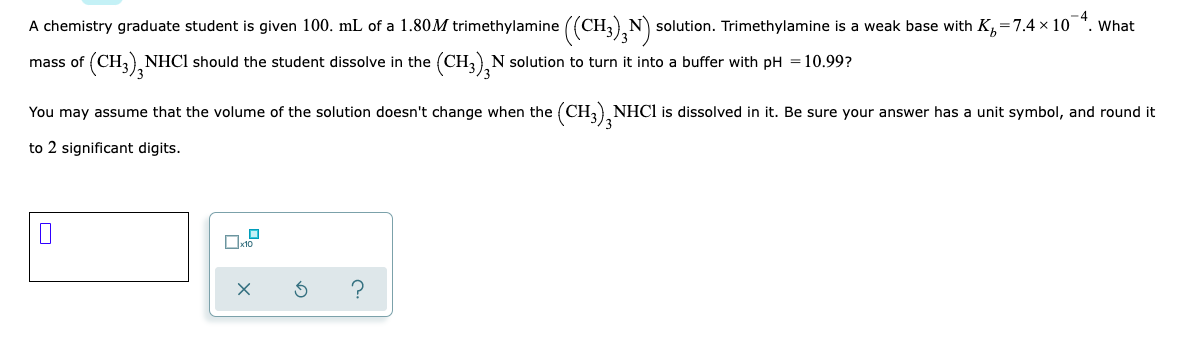
Transcribed Image Text:A chemistry graduate student is given 100. mL of a 1.80M trimethylamine ((CH N solution. Trimethylamine is a weak base with K,=7.4 x 10 *. What
(CH3), NHC1 should the student dissolve in the
(CH3),N solution to turn it into a buffer with pH =10.99?
mass of
You may assume that the volume of the solution doesn't change when the (CH, NHCI is dissolved in it. Be sure your answer has a unit symbol, and round it
to 2 significant digits.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
