A commercial airliner has an internal pressure of 1.00 atm and temperature of 25° C at takeoff. If the temperature of the airliner drops to 17° C during the flight, what is the new cabin pressure? (assume constant volume) A sample of What is the molar mass of the gas? If the unknown gas is composed of 0.815 g of carbon and the rest is hydrogen, what is its with a mass of 1.020 g occupies a volume of 762 mL at 0 °C and 1.00 atm (STP). gas molecular formula?
A commercial airliner has an internal pressure of 1.00 atm and temperature of 25° C at takeoff. If the temperature of the airliner drops to 17° C during the flight, what is the new cabin pressure? (assume constant volume) A sample of What is the molar mass of the gas? If the unknown gas is composed of 0.815 g of carbon and the rest is hydrogen, what is its with a mass of 1.020 g occupies a volume of 762 mL at 0 °C and 1.00 atm (STP). gas molecular formula?
Living By Chemistry: First Edition Textbook
1st Edition
ISBN:9781559539418
Author:Angelica Stacy
Publisher:Angelica Stacy
ChapterU3: Weather: Phase Changes And Behaviour Of Gases
Section: Chapter Questions
Problem 9RE
Related questions
Question
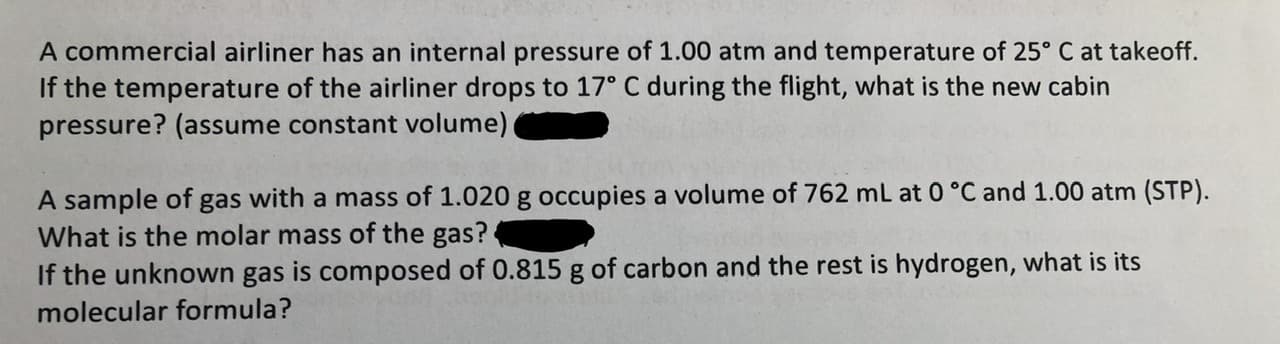
Transcribed Image Text:A commercial airliner has an internal pressure of 1.00 atm and temperature of 25° C at takeoff.
If the temperature of the airliner drops to 17° C during the flight, what is the new cabin
pressure? (assume constant volume)
A sample of
What is the molar mass of the gas?
If the unknown gas is composed of 0.815 g of carbon and the rest is hydrogen, what is its
with a mass of 1.020 g occupies a volume of 762 mL at 0 °C and 1.00 atm (STP).
gas
molecular formula?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 7 steps with 4 images

Recommended textbooks for you

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning