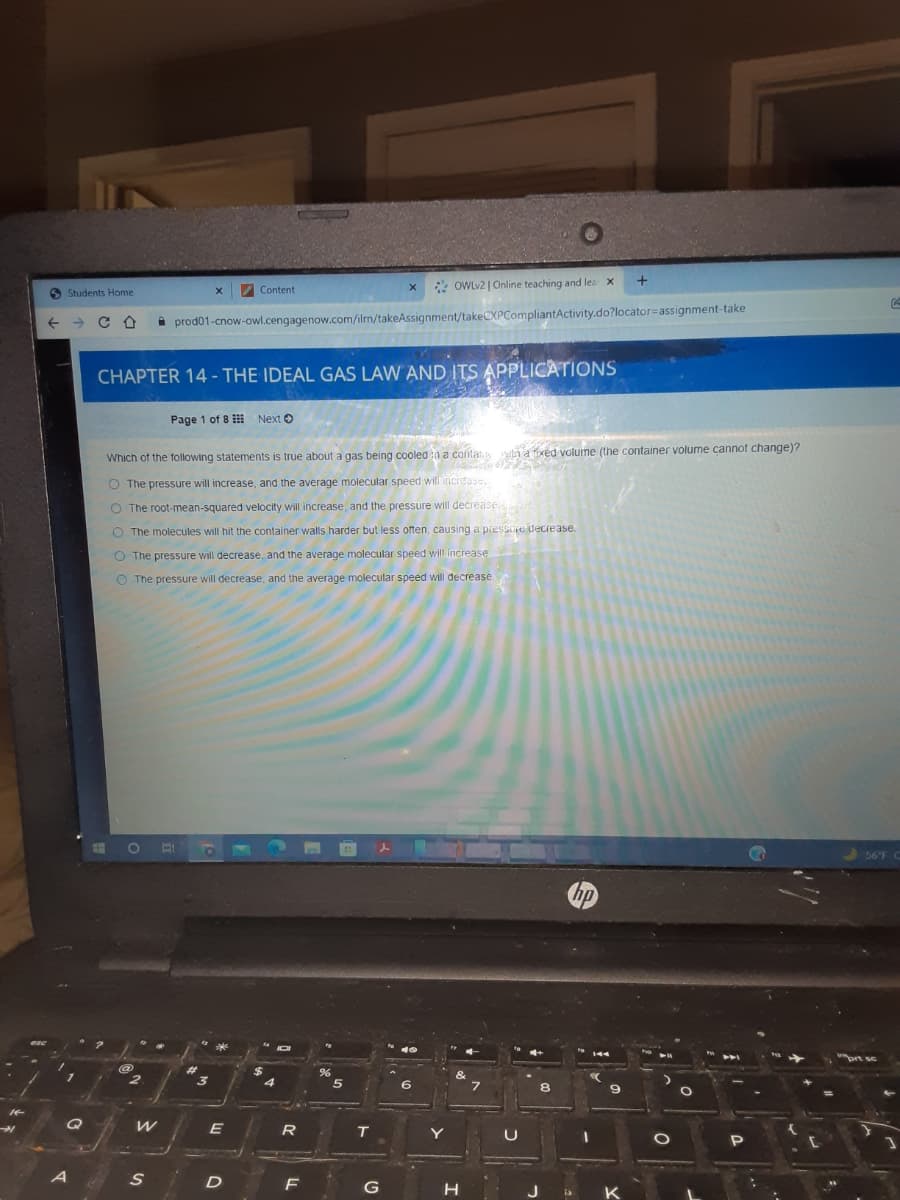
Which of the following statements is true about a gas being cooled n a conta win a fxed volume (the container volume cannot change)? O The pressure will increase, and the average molecular speed will incidase. O The root-mean-squared velocity will increase, and the pressure will decrease O The molecules will hit the container walls harder but less often, causing a piesse decrease. The pressure will decrease, and the average molecular speed will increase The pressure will decrease, and the average molecular speed will decrease
Which of the following statements is true about a gas being cooled n a conta win a fxed volume (the container volume cannot change)? O The pressure will increase, and the average molecular speed will incidase. O The root-mean-squared velocity will increase, and the pressure will decrease O The molecules will hit the container walls harder but less often, causing a piesse decrease. The pressure will decrease, and the average molecular speed will increase The pressure will decrease, and the average molecular speed will decrease
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter5: The Gaseous State
Section: Chapter Questions
Problem 5.127QP: A 1.000-g sample of an unknown gas at 0C gives the following data: P(atm) V (L) 0.2500 3.1908 0.5000...
Related questions
Question
Transcribed Image Text:a xed volume (the container volume cannot change)?
Content
* OWLV2 | Online teaching and lea x
O Students Home
i prod01-cnow-owl.cengagenow.com/ilm/takeAssignment/takeCXPCompliantActivity.do?locator=assignment-take
CHAPTER 14 - THE IDEAL GAS LAW AND ITS APPLICATIONS
Page 1 of 8 Next O
Which of the following statements is true about a gas being cooled n a conla
O The pressure will increase, and the average molecular speed will incidase.
O The root-mean-squared velocity will increase, and the pressure will decrease
O The molecules will hit the container walls harder but less often, causing a pressaje decrease.
O The pressure will decrease, and the average molecular speed will increase.
O The pressure will decrease, and the average molecular speed will decrease
56'F
144
@
6.
8.
R
T
Y
F
G
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,