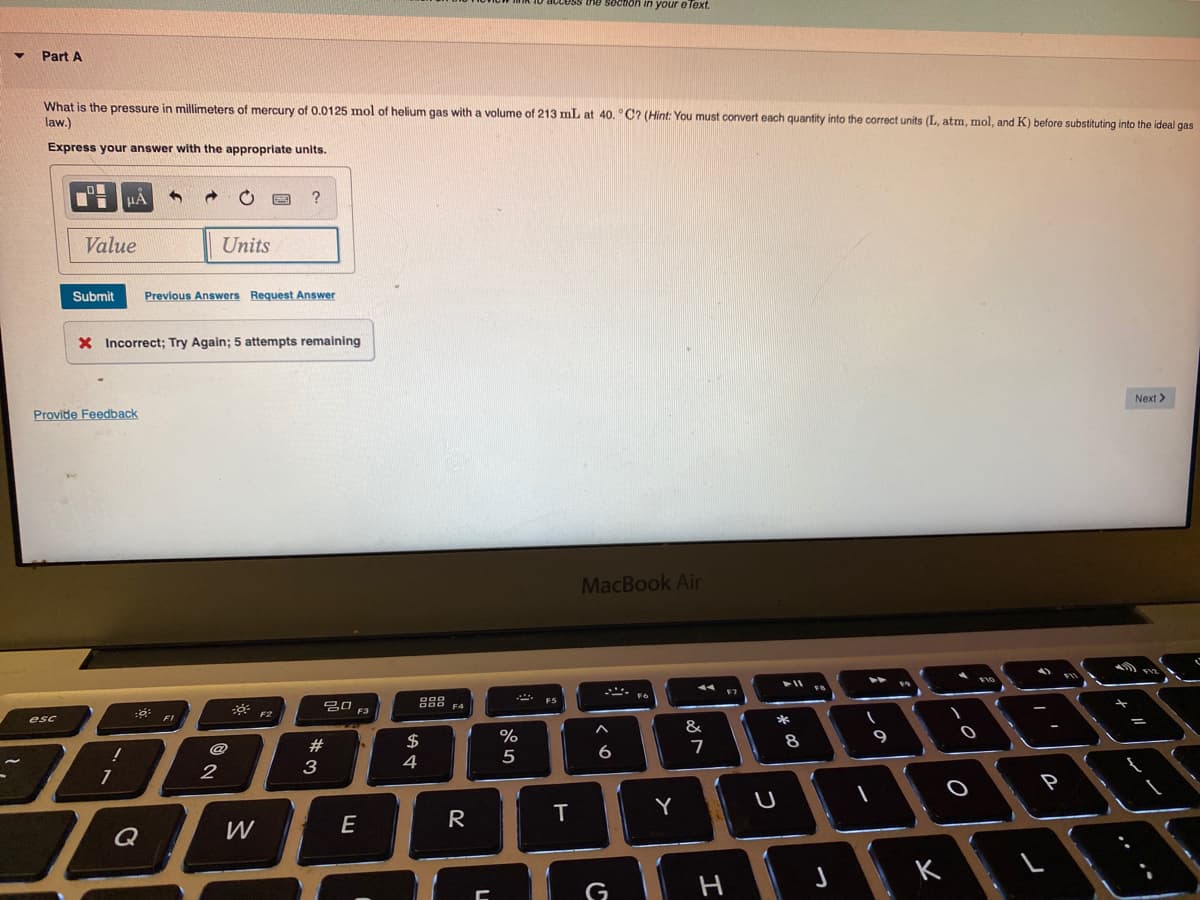
What is the pressure in millimeters of mercury of 0.0125 mol of helium gas with a volume of 213 mL at 40. °C? (Hint: You must convert each quantity into the correct units (L, atm, mol, and K) before substituting into the ideal gas law.) Express your answer with the appropriate units. ? Value Units
What is the pressure in millimeters of mercury of 0.0125 mol of helium gas with a volume of 213 mL at 40. °C? (Hint: You must convert each quantity into the correct units (L, atm, mol, and K) before substituting into the ideal gas law.) Express your answer with the appropriate units. ? Value Units
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter10: Gases And Their Properties
Section: Chapter Questions
Problem 56PS: Consider a 5.00-L tank containing 375 g of Ar at a temperature of 25 C. (a) Calculate the pressure...
Related questions
Question
Transcribed Image Text:CLESS the soction in your e lext.
Part A
What is the pressure in millimeters of mercury of 0.0125 mol of helium gas with a volume of 213 mL at 40. °C? (Hint: You must convert each quantity into the correct units (L, atm, mol, and K) before substituting into the ideal gas
law.)
Express your answer with the appropriate units.
Value
Units
Submit
Previous Answers Request Answer
X Incorrect; Try Again; 5 attempts remalining
Next >
Provide Feedback
MacBook Air
F7
吕口
F3
esc
*
&
$
%
9
@
#
6
3
4
P
T
Y
E
R
Q
G
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning
