A common rule-of-thumb for many chemical reactions states that, at normal conditions, the reaction rate doubles for each increase of 10°C. Assuming a "normal" temperature of 20°C, estimate the activation energy of a "typical" chemical reaction. If we select 20°C as the reference temperature, what is a "typical" dimensionless activation energy?
A common rule-of-thumb for many chemical reactions states that, at normal conditions, the reaction rate doubles for each increase of 10°C. Assuming a "normal" temperature of 20°C, estimate the activation energy of a "typical" chemical reaction. If we select 20°C as the reference temperature, what is a "typical" dimensionless activation energy?
Chapter8: Sampling, Standardization, And Calibration
Section: Chapter Questions
Problem 8.19QAP
Related questions
Question
100%
Please solve this question
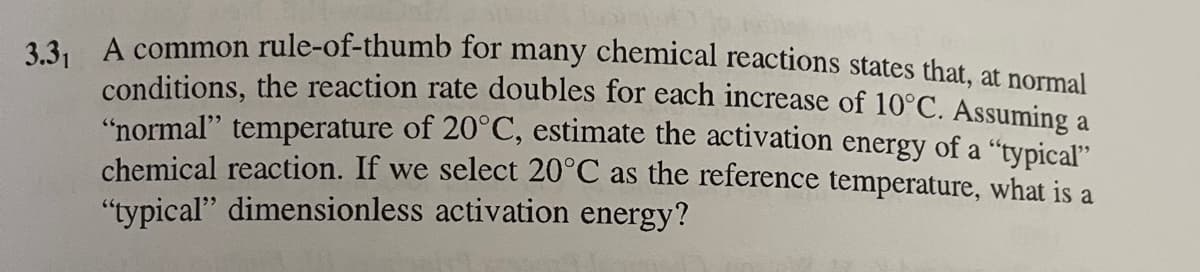
Transcribed Image Text:3.3, A common rule-of-thumb for many chemical reactions states that, at normal
conditions, the reaction rate doubles for each increase of 10°C. Assuming a
"normal" temperature of 20°C, estimate the activation energy of a "typical"
chemical reaction. If we select 20°C as the reference temperature, what is a
"typical" dimensionless activation energy?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



