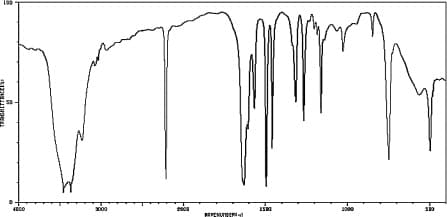
A compound's IR spectrum is given below. (a) Indicate the presence of at least 4 bonds/functional groups based on the IR spectrum. For each bond/functional group, either mark its peak on the spectrum or give the frequency of the peak in the spectrum. (b) Indicate the absence of at least 3 bonds/functional groups based on the IR spectrum. For each bond/functional group, either mark where its peak on the spectrum should appear (but doesn't) or give the frequency range where you would expect it to appear. (c) What is the structure of the compound? The molecular formula is C7H6N2. (d) Explain how you determined the structure of the compound.
A compound's IR spectrum is given below. (a) Indicate the presence of at least 4 bonds/
*There's no more additional informations for this problem*
Step by step
Solved in 5 steps with 6 images




