A cup containing 93.0 ml of H20 is initially at room temperature 23.0°C. A chilled steel rod at 2.4°C is placed in the water. If the final temperature of the system is 22.2°C, what is the mass of the steel rod? Use the following values: density of water = 1.00 g ml specific heat capacity of water = 4.18 Jg-1 oc-1 specific heat capacity of steel = 0.452 ) g1 oc-1 Express your answer to three significant figures. mass of steel rod = 9. Check
A cup containing 93.0 ml of H20 is initially at room temperature 23.0°C. A chilled steel rod at 2.4°C is placed in the water. If the final temperature of the system is 22.2°C, what is the mass of the steel rod? Use the following values: density of water = 1.00 g ml specific heat capacity of water = 4.18 Jg-1 oc-1 specific heat capacity of steel = 0.452 ) g1 oc-1 Express your answer to three significant figures. mass of steel rod = 9. Check
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter4: Energy And Chemical Reactions
Section4.4: Heat Capacity
Problem 4.3PSP: A piece of aluminum with a mass of 250. g is at an initial temperature of 5.0 C. If 24.1 kJ is...
Related questions
Question
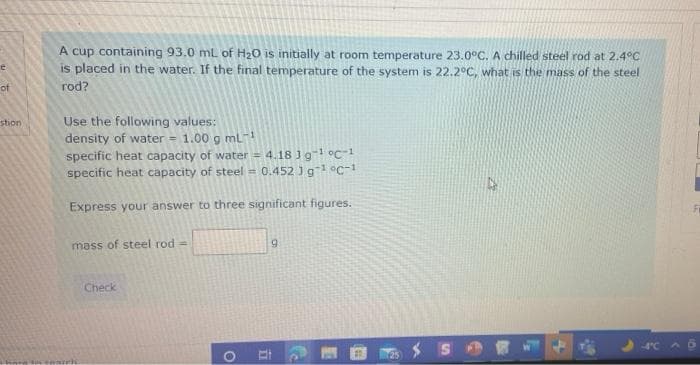
Transcribed Image Text:A cup containing 93.0 mL of H20 is initially at room temperature 23.0°C. A chilled steel rod at 2.4°C
is placed in the water. If the final temperature of the system is 22.2°C, what is the mass of the steel
of
rod?
Use the following values:
density of water = 1.00 g ml-
stion
4.18 Jg-1 °c-1
specific heat capacity of water =
specific heat capacity of steel = 0.452 J g °c-1
Express your answer to three significant figures.
mass of steel rod =
Check
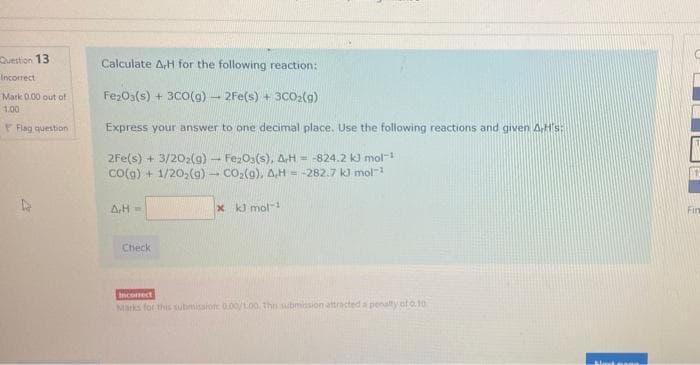
Transcribed Image Text:Question 13
Calculate A,H for the following reaction:
Incorrect
Mark 0.00 out of
FezO3(s) + 3CO(g) - 2Fe(s) + 3CO2(g)
1.00
Flag question
Express your answer to one decimal place. Use the following reactions and given AH's:
2Fe(s) + 3/2O2(9) - FezO:(s), AH = -824.2 k) mol-
CO(g) + 1/20,(g) - Co,(a), A,H = -282.7 k) mol-
AH
x kJ mol-
Fin
Check
comect
Marks for this submission 0.00/1.00. ths submission attracted a penalty oto10
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning