0.2 0.5×10-4 1x10-4 1.5x10-4 2x10-4 [Fe(SCN2+)]equ, (M) y = 4.26442 × 103 x + 0.0202852 R2 = 0.999902 In the laboratory you measured RMSE, however here you will enter the R2 value displayed on the graph. SLOPE, (M-1) INTERCEPT R2 Unrounded 4.26442 x 103 0.0202852 0.999902 Rounded 4.264 x 103 0.02029 0.9999 CALCULATED VALUE • The path length of the vial is 1.00 cm. Unrounded Rounded Molar absorption coefficient for Fe(SCN)²+, (M-1 cm-1) 3
0.2 0.5×10-4 1x10-4 1.5x10-4 2x10-4 [Fe(SCN2+)]equ, (M) y = 4.26442 × 103 x + 0.0202852 R2 = 0.999902 In the laboratory you measured RMSE, however here you will enter the R2 value displayed on the graph. SLOPE, (M-1) INTERCEPT R2 Unrounded 4.26442 x 103 0.0202852 0.999902 Rounded 4.264 x 103 0.02029 0.9999 CALCULATED VALUE • The path length of the vial is 1.00 cm. Unrounded Rounded Molar absorption coefficient for Fe(SCN)²+, (M-1 cm-1) 3
Chapter10: Effect Of Electrolytes On Chemical Equilibria
Section: Chapter Questions
Problem 10.16QAP
Related questions
Question
I need help calculating the molar absorption please!
![0.2
0.5×10-4
1x10-4
1.5x10-4
2x10-4
[Fe(SCN2+)]equ, (M)
y = 4.26442 × 103 x + 0.0202852
R2 = 0.999902
In the laboratory you measured RMSE, however here you will enter the R2 value displayed on the graph.
SLOPE, (M-1)
INTERCEPT
R?
Unrounded
4.26442 x 10³
0.0202852
0.999902
Rounded
4.264 x 103
0.02029
0.9999
CALCULATED VALUE
• The path length of the vial is 1.00 cm.
Unrounded
Rounded
Molar absorption coefficient for Fe(SCN), (M-1 cm-1)
3
4](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F124af7df-215c-470f-a3a7-f7a2bdab4b3f%2Fc9bef9d1-8f1e-4ca6-917d-6c5fda049d9f%2F5nkuash_processed.png&w=3840&q=75)
Transcribed Image Text:0.2
0.5×10-4
1x10-4
1.5x10-4
2x10-4
[Fe(SCN2+)]equ, (M)
y = 4.26442 × 103 x + 0.0202852
R2 = 0.999902
In the laboratory you measured RMSE, however here you will enter the R2 value displayed on the graph.
SLOPE, (M-1)
INTERCEPT
R?
Unrounded
4.26442 x 10³
0.0202852
0.999902
Rounded
4.264 x 103
0.02029
0.9999
CALCULATED VALUE
• The path length of the vial is 1.00 cm.
Unrounded
Rounded
Molar absorption coefficient for Fe(SCN), (M-1 cm-1)
3
4
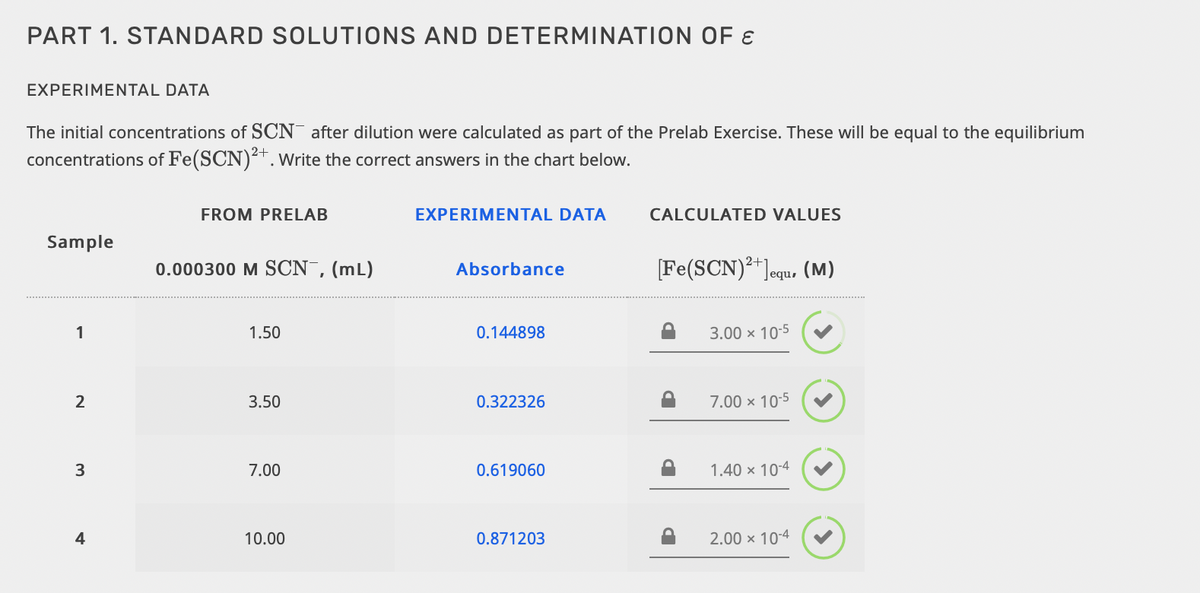
Transcribed Image Text:PART 1. STANDARD SOLUTIONS AND DETERMINATION OF E
EXPERIMENTAL DATA
The initial concentrations of SCN after dilution were calculated as part of the Prelab Exercise. These will be equal to the equilibrium
concentrations of Fe(SCN)². Write the correct answers in the chart below.
FROM PRELAB
EXPERIMENTAL DATA
CALCULATED VALUES
Sample
0.000300 M SCN,(mL)
[Fe(SCN)**Jequ, (M)
Absorbance
1
1.50
0.144898
3.00 x 10-5
2
3.50
0.322326
7.00 x 10-5
3
7.00
0.619060
1.40 x 10-4
4
10.00
0.871203
2.00 × 10-4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning