(a) For a sample of gas at constant temperature, its pressure multiplied by its volume is a constant. (b) For a sample of gas at constant temperature, in- creasing the pressure increases the volume. (c) For a sample of gas at constant temperature, P,/V, = P,/V,.
(a) For a sample of gas at constant temperature, its pressure multiplied by its volume is a constant. (b) For a sample of gas at constant temperature, in- creasing the pressure increases the volume. (c) For a sample of gas at constant temperature, P,/V, = P,/V,.
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter5: Gases, Liquids, And Solids
Section: Chapter Questions
Problem 5.59P
Related questions
Question
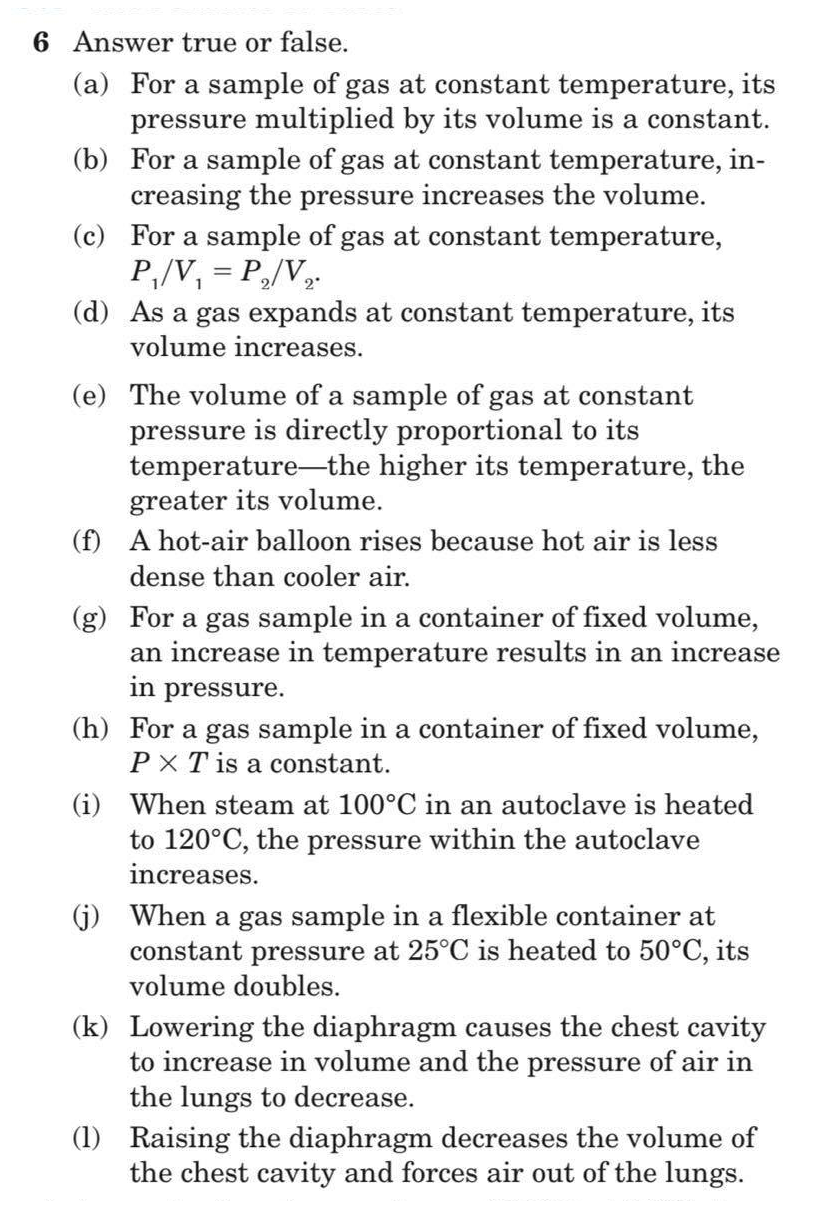
Transcribed Image Text:6 Answer true or false.
(a) For a sample of gas at constant temperature, its
pressure multiplied by its volume is a constant.
(b) For a sample of gas at constant temperature, in-
creasing the pressure increases the volume.
(c) For a sample of gas at constant temperature,
P,/V, = P,/V,.
(d) As a gas expands at constant temperature, its
volume increases.
(e) The volume of a sample of gas at constant
pressure is directly proportional to its
temperature-the higher its temperature, the
greater its volume.
(f) A hot-air balloon rises because hot air is less
dense than cooler air.
(g) For a gas sample in a container of fixed volume,
an increase in temperature results in an increase
in pressure.
(h) For a gas sample in a container of fixed volume,
PX T is a constant.
(i) When steam at 100°C in an autoclave is heated
to 120°C, the pressure within the autoclave
reases.
(j) When a gas sample in a flexible container at
constant pressure at 25°C is heated to 50°C, its
volume doubles.
(k) Lowering the diaphragm causes the chest cavity
to increase in volume and the pressure of air in
the lungs to decrease.
(1) Raising the diaphragm decreases the volume of
the chest cavity and forces air out of the lungs.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,