A galvanic cell is constructed of two half cells Zn2+*(aq)/Zn(s) and H2(g)/H*(aq) separated by a salt bridge. When [Zn²*] = 1.0 M and P(H2) = 1.0 atm, the emf of this cell is found to be E = 0.685 V at %3D %3D 25 °C. Use standard electrode potential p. 901 Tro textbook 4th Ed. a) Write a balanced redox reaction that occurs in the galvanic cell. b) Indicate the half reaction that happens in each half cell. c) Indicate the anode AND the cathode
A galvanic cell is constructed of two half cells Zn2+*(aq)/Zn(s) and H2(g)/H*(aq) separated by a salt bridge. When [Zn²*] = 1.0 M and P(H2) = 1.0 atm, the emf of this cell is found to be E = 0.685 V at %3D %3D 25 °C. Use standard electrode potential p. 901 Tro textbook 4th Ed. a) Write a balanced redox reaction that occurs in the galvanic cell. b) Indicate the half reaction that happens in each half cell. c) Indicate the anode AND the cathode
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 76AP
Related questions
Question
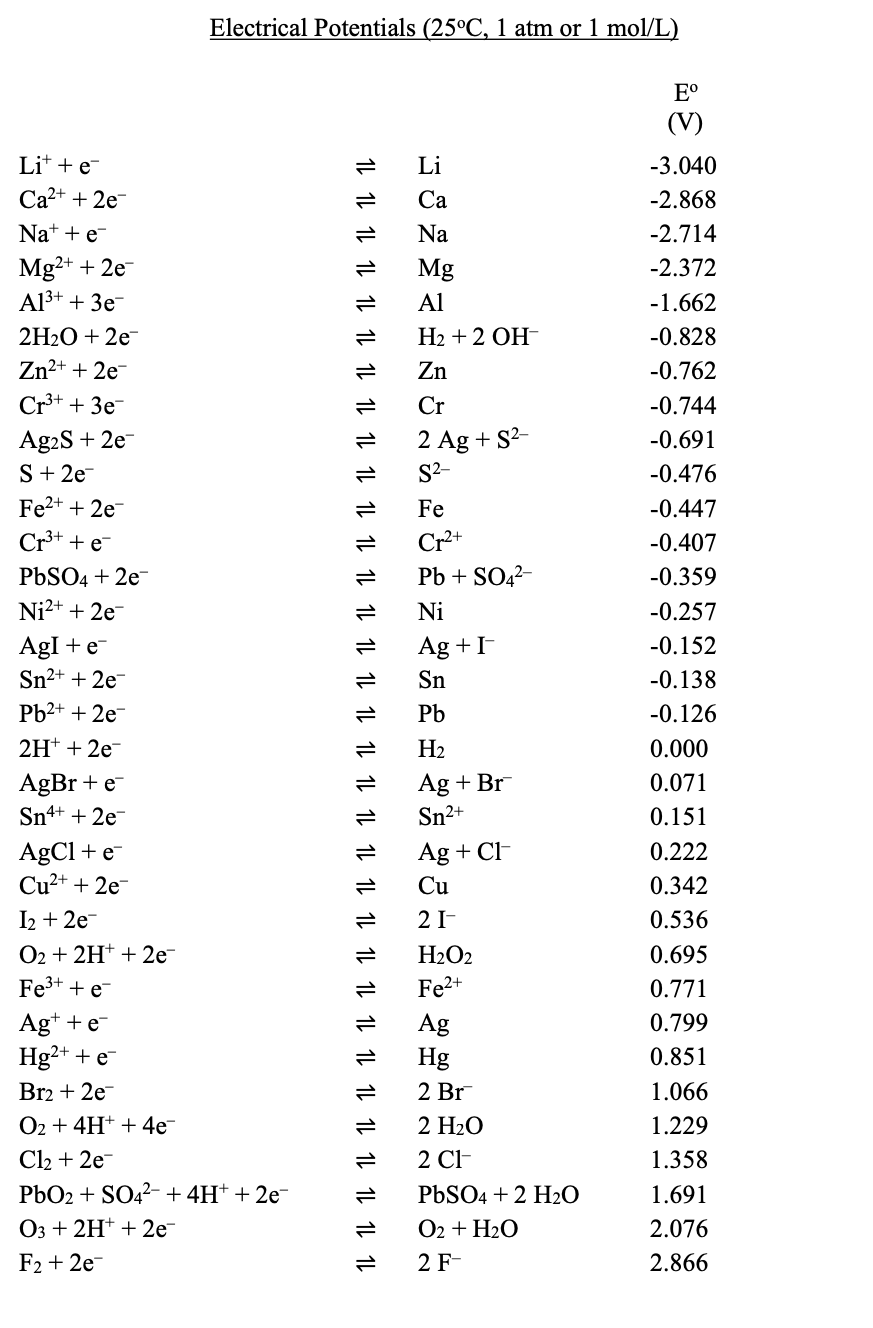
Transcribed Image Text:Electrical Potentials (25°C, 1 atm or 1 mol/L)
E°
(V)
Lit + e-
Li
-3.040
Ca2+ + 2e
Са
-2.868
Na* + e-
Na
-2.714
Mg2+ + 2e-
A13+ + 3e-
Mg
-2.372
Al
-1.662
2H20 + 2е-
H2 + 2 OH-
-0.828
Zn2+ + 2e
Zn
-0.762
Cr+ + 3e-
Cr
-0.744
Ag2S + 2e-
S+ 2e
Fe2+ + 2e-
2 Ag + S?-
S2-
-0.691
-0.476
Fe
-0.447
Cr** +e
Cr2+
-0.407
PBSO4 + 2e-
Pb + SO42-
-0.359
Ni2+ + 2e-
Ni
-0.257
AgI +e-
Sn²+ + 2e-
Ag +I
-0.152
Sn
-0.138
Pb2+ + 2e
Pb
-0.126
2H* + 2e
H2
0.000
AgBr + e-
Sn4+ + 2e
Ag + Br
Sn2+
0.071
0.151
AgCl + e-
Cu2+ + 2e
Ag + CH
0.222
Cu
0.342
I2 + 2e-
O2 + 2H* + 2e
Fe3+ + e
2 I-
0.536
H2O2
0.695
Fe2+
0.771
Ag+ +e-
Hg²+ + e-
0.799
Ag
Hg
0.851
Br2 + 2e
2 Br
1.066
O2 + 4H+ + 4e
2 H2O
1.229
Cl2 + 2e
PbO2 + SO42- + 4H+ + 2e-
O3 + 2H* + 2e
2 C-
1.358
PBSO4 + 2 H20
O2 + H20
2 F-
1.691
2.076
F2 + 2e-
2.866
1L 1L 11 1L 1L 1L 1L 1L 11 1L 1L 1L 1L
1L 1L 1L 1L 11 1L 1L 1L 1
1L 1L 1L 1L 11 1L 11
![A galvanic cell is constructed of two half cells Zn2*(aq)/Zn(s) and H2(g)/H*(aq) separated by a salt
bridge. When [Zn2*] = 1.0 M and P(H2) = 1.0 atm, the emf of this cell is found to be E = 0.685 V at
%3D
25 °C.
Use standard electrode potential p. 901 Tro textbook 4th Ed.
a) Write a balanced redox reaction that occurs in the galvanic cell.
b) Indicate the half reaction that happens in each half cell.
c) Indicate the anode AND the cathode
d) Calculate the H*(aq) concentration.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fbe7921cf-39aa-4ed3-ba83-099ed11a60d1%2F1d8a3149-867e-45b0-9775-855ab165f273%2F9y0xzmw_processed.png&w=3840&q=75)
Transcribed Image Text:A galvanic cell is constructed of two half cells Zn2*(aq)/Zn(s) and H2(g)/H*(aq) separated by a salt
bridge. When [Zn2*] = 1.0 M and P(H2) = 1.0 atm, the emf of this cell is found to be E = 0.685 V at
%3D
25 °C.
Use standard electrode potential p. 901 Tro textbook 4th Ed.
a) Write a balanced redox reaction that occurs in the galvanic cell.
b) Indicate the half reaction that happens in each half cell.
c) Indicate the anode AND the cathode
d) Calculate the H*(aq) concentration.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning