In a galvanic cell, the cathode consists of a Ag*(1.00 M)| Ag half-cell. The anode is a platinum wire, with hydrogen bub- bling over it at 1.00-atm pressure, which is immersed in a buffer solution containing benzoic acid and sodium benzo- ate. The concentration of benzoic acid (CH;COOH) is 0.10 M, and that of benzoate ion (C,H;COO") is 0.050 M. The overall cell reaction is then Ag* (aq) + H2(g) + H20(€) → Ag(s) + H;O* (aq) and the measured cell potential is 1.030 V. Calculate the pH in the buffer solution and determine the K, of benzoic acid.
In a galvanic cell, the cathode consists of a Ag*(1.00 M)| Ag half-cell. The anode is a platinum wire, with hydrogen bub- bling over it at 1.00-atm pressure, which is immersed in a buffer solution containing benzoic acid and sodium benzo- ate. The concentration of benzoic acid (CH;COOH) is 0.10 M, and that of benzoate ion (C,H;COO") is 0.050 M. The overall cell reaction is then Ag* (aq) + H2(g) + H20(€) → Ag(s) + H;O* (aq) and the measured cell potential is 1.030 V. Calculate the pH in the buffer solution and determine the K, of benzoic acid.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 14P
Related questions
Question
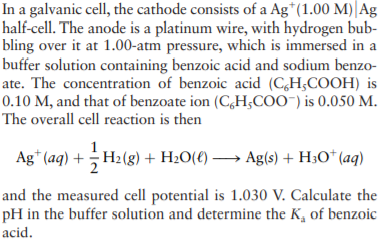
Transcribed Image Text:In a galvanic cell, the cathode consists of a Ag*(1.00 M)| Ag
half-cell. The anode is a platinum wire, with hydrogen bub-
bling over it at 1.00-atm pressure, which is immersed in a
buffer solution containing benzoic acid and sodium benzo-
ate. The concentration of benzoic acid (CH;COOH) is
0.10 M, and that of benzoate ion (C,H;COO") is 0.050 M.
The overall cell reaction is then
Ag* (aq) + H2(g) + H20(€) → Ag(s) + H;O* (aq)
and the measured cell potential is 1.030 V. Calculate the
pH in the buffer solution and determine the K, of benzoic
acid.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 6 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning