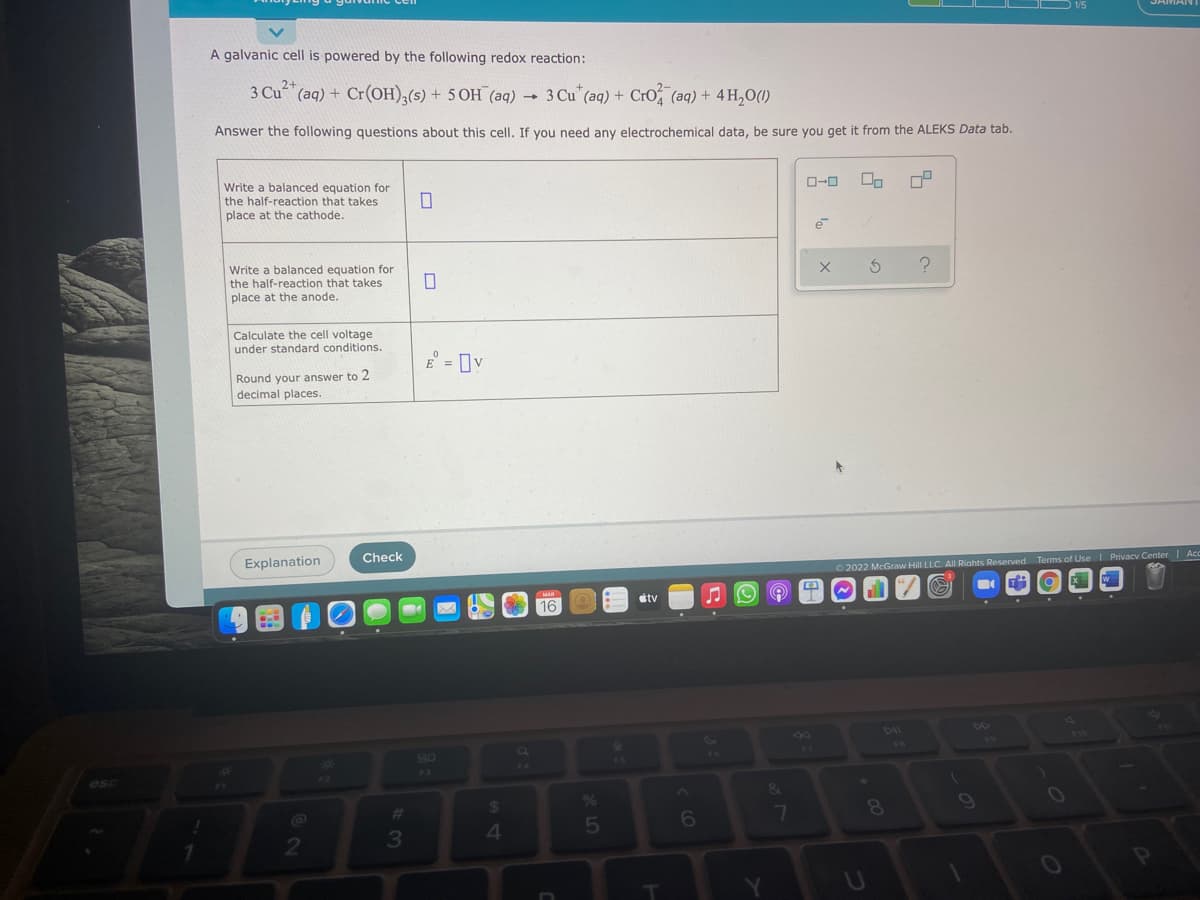
A galvanic cell is powered by the following redox reaction: 3 Cu 3 Cu" (aq)+ Cro, (aq) + 4 H,0(1) (aq) + Cr(OH),(s) + 5 OH (aq) Answer the following questions about this cell. If you need any electrochemical data, be sure you get it from the ALEKS Data tab. O-0 Write a balanced equation for the half-reaction that takes place at the cathode. Write a balanced equation for the half-reaction that takes place at the anode. Calculate the cell voltage under standard conditions. E = Ov %3D Round your answer to 2 decimal places.
A galvanic cell is powered by the following redox reaction: 3 Cu 3 Cu" (aq)+ Cro, (aq) + 4 H,0(1) (aq) + Cr(OH),(s) + 5 OH (aq) Answer the following questions about this cell. If you need any electrochemical data, be sure you get it from the ALEKS Data tab. O-0 Write a balanced equation for the half-reaction that takes place at the cathode. Write a balanced equation for the half-reaction that takes place at the anode. Calculate the cell voltage under standard conditions. E = Ov %3D Round your answer to 2 decimal places.
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter19: Principles Of Chemical Reactivity: Electron Transfer Reactions
Section19.9: Corrosion: Redox Reactions In The Environment
Problem 2.5ACP: Assume the following electrochemical cell simulates the galvanic cell formed by copper and zinc in...
Related questions
Question
Transcribed Image Text:1/5
A galvanic cell is powered by the following redox reaction:
3 Cu2+
(aq) + Cr(OH),(s) + 5 OH (aq)
3Cu"(aq) + CrO (aq) + 4 H,0(1)
Answer the following questions about this cell. If you need any electrochemical data, be sure you get it from the ALEKS Data tab.
Write a balanced equation for
the half-reaction that takes
O-0
place at the cathode.
Write a balanced equation for
the half-reaction that takes
place at the anode.
Calculate the cell voltage
under standard conditions.
E = Iv
Round your answer to 2
decimal places.
Explanation
Check
2022 McGraw Hill LLC. All Riahts Reserved Terms of UseI Privacv Center Acc
MAR
etv
16
..
Dl
DO
3
44
110
80
F6
esc
F5
%23
24
%
10
4.
5
6
7
U
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning