A gas consisting only of CO and N2 is made by passing a mixture of flue gas and air through a bed of incandescent coke (assume pure carbon). The two reactions that occur both go to completion: CO2 + C-2CO AH,,298 172.46 kJ/mol AH,298-221.05 kJ/mol AH. 2C+02-200 The flue gas composition is 12.8 mol % CO, 3.7 mol % CO2, 5.4 mol % O2, and 78.1 mol % N₂. The flue gas/air mixture is so proportioned that the heats of the two reactions cancel. If the feed stream is preheated to 875 °C, and the temperature of the coke bed is therefore constant at 875 °C. If the process is adiabatic, what ratio of moles of flue gas to moles of air is required. Pulto Mixer Coke Flue gas Air
A gas consisting only of CO and N2 is made by passing a mixture of flue gas and air through a bed of incandescent coke (assume pure carbon). The two reactions that occur both go to completion: CO2 + C-2CO AH,,298 172.46 kJ/mol AH,298-221.05 kJ/mol AH. 2C+02-200 The flue gas composition is 12.8 mol % CO, 3.7 mol % CO2, 5.4 mol % O2, and 78.1 mol % N₂. The flue gas/air mixture is so proportioned that the heats of the two reactions cancel. If the feed stream is preheated to 875 °C, and the temperature of the coke bed is therefore constant at 875 °C. If the process is adiabatic, what ratio of moles of flue gas to moles of air is required. Pulto Mixer Coke Flue gas Air
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter5: Thermochemistry
Section: Chapter Questions
Problem 5.108QE
Related questions
Question
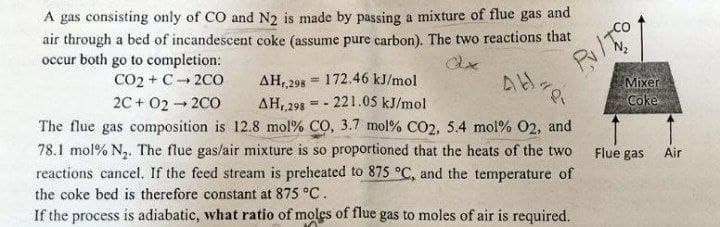
Transcribed Image Text:A gas consisting only of CO and N2 is made by passing a mixture of flue gas and
air through a bed of incandescent coke (assume pure carbon). The two reactions that
occur both go to completion:
CO2 + C-2CO
h
AH,,298 172.46 kJ/mol
- 221.05 kJ/mol
--
ΔΗ,,298
The flue gas composition is 12.8 mol% CO, 3.7 mol % CO2, 5.4 mol % O2, and
78.1 mol % N₂. The flue gas/air mixture is so proportioned that the heats of the two
reactions cancel. If the feed stream is preheated to 875 °C, and the temperature of
the coke bed is therefore constant at 875 °C.
If the process is adiabatic, what ratio of moles of flue gas to moles of air is required.
2C+02 → 2CO
-
=
AH.
Pl
Mixer
Coke
Flue gas
Air
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning