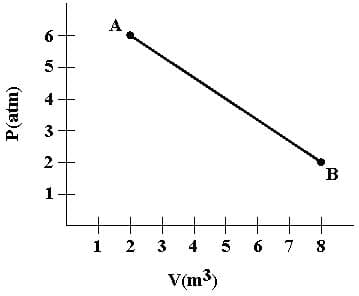
A gas expands as shown. If the heat taken in during this process is 1.02x106 J and 1 atm = 1.01x105 N.m-2, the change in internal energy of the gas is
A gas expands as shown. If the heat taken in during this process is 1.02x106 J and 1 atm = 1.01x105 N.m-2, the change in internal energy of the gas is
College Physics
11th Edition
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Raymond A. Serway, Chris Vuille
Chapter16: Electrical Energy And Capacitance
Section: Chapter Questions
Problem 25P: Calculate the speed of (a) an electron and (b) a proton with a kinetic energy of 1.00 electron volt...
Related questions
Question
100%
A gas expands as shown. If the heat taken in during this process is 1.02x106 J and 1 atm = 1.01x105 N.m-2, the change in internal energy of the gas is
Transcribed Image Text:A
5
4
2
в
B
1
1 2 3 4 5 6 7 8
V(m3)
6
3.
P(atm)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning