A gaseous mixture of O2 and N2 contains 30.8 % nitrogen by mass. What is the partial pressure of oxygen in the mixture if the total pressure is 725 mmHg ? Express you answer numerically in millimeters of mercury. View Available Hint(s) ΑΣφ 502 Poxygen mmHg охуg Previous Answers Submit Incorrect; Try Again; 3 attempts remaining Your answer would be correct if the given percentage was "by moles." Since the given percentage is "by mass," you will have to calculate the moles and the mole fraction. You may want to review Hint 1. How to approach the problem.
A gaseous mixture of O2 and N2 contains 30.8 % nitrogen by mass. What is the partial pressure of oxygen in the mixture if the total pressure is 725 mmHg ? Express you answer numerically in millimeters of mercury. View Available Hint(s) ΑΣφ 502 Poxygen mmHg охуg Previous Answers Submit Incorrect; Try Again; 3 attempts remaining Your answer would be correct if the given percentage was "by moles." Since the given percentage is "by mass," you will have to calculate the moles and the mole fraction. You may want to review Hint 1. How to approach the problem.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter9: The Gaseous State
Section: Chapter Questions
Problem 6P
Related questions
Question
100%
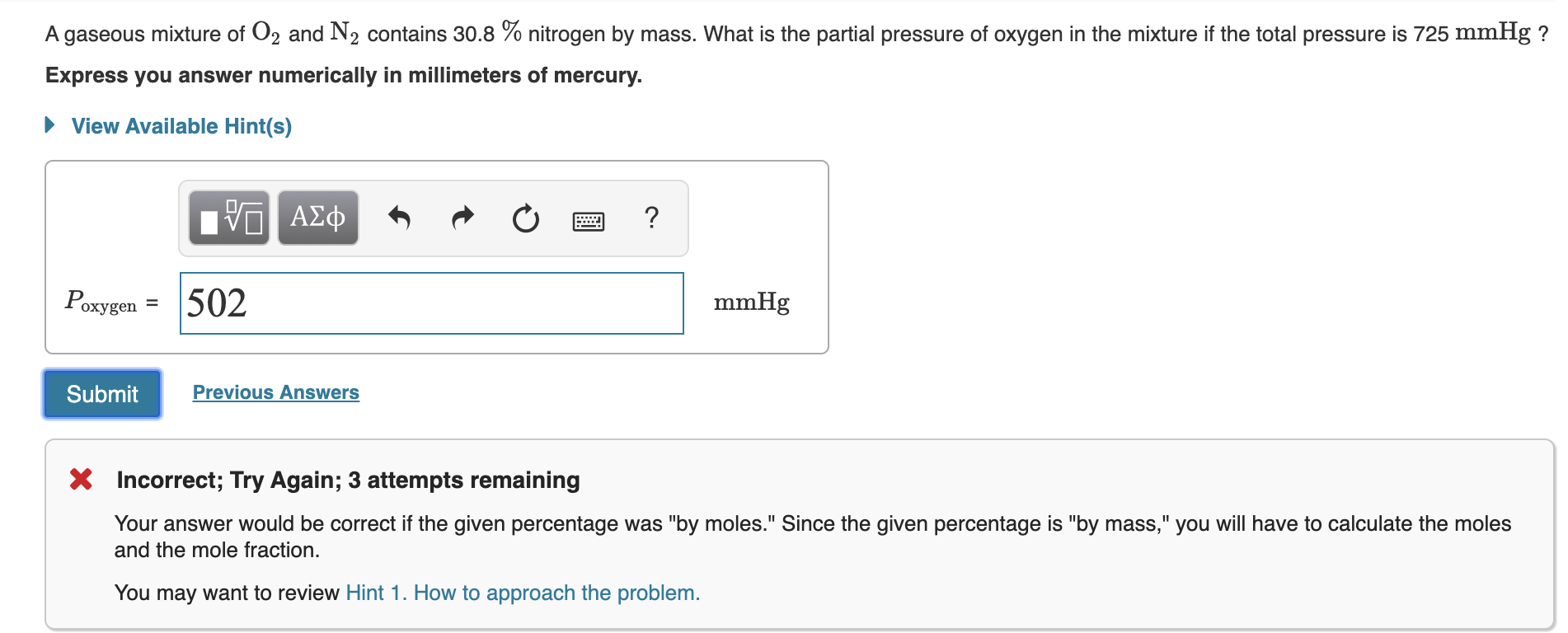
Transcribed Image Text:A gaseous mixture of O2 and N2 contains 30.8 % nitrogen by mass. What is the partial pressure of oxygen in the mixture if the total pressure is 725 mmHg ?
Express you answer numerically in millimeters of mercury.
View Available Hint(s)
ΑΣφ
502
Poxygen
mmHg
охуg
Previous Answers
Submit
Incorrect; Try Again; 3 attempts remaining
Your answer would be correct if the given percentage was "by moles." Since the given percentage is "by mass," you will have to calculate the moles
and the mole fraction.
You may want to review Hint 1. How to approach the problem.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 3 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,