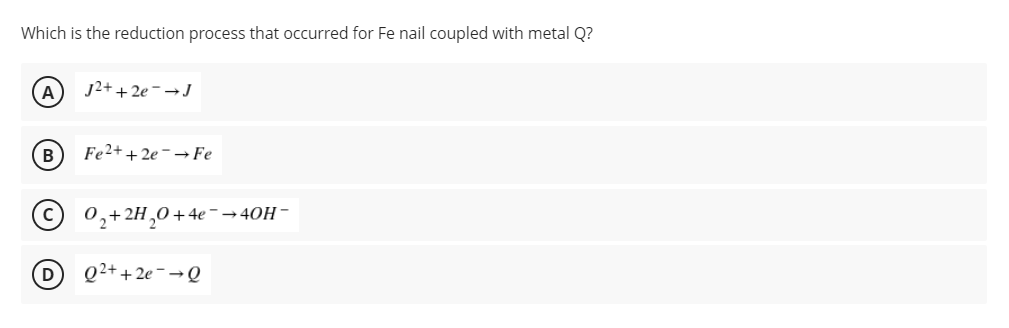
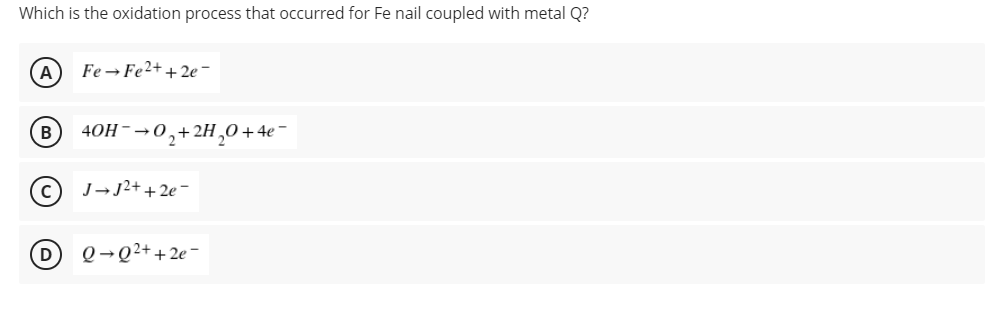
A group of students were tasked to perform the metal coupling of iron nail similar to Experiment 3: Corrosion of Metals. They were given two metals, metal Q and metal J, to couple to iron nail. They prepared an agar medium with K3Fe(CN)6 and phenolphthalein. Their professor informed them that metal Q forms an orange color while metal J forms maroon color when oxidized. Their professors also gave them the reduction half-reactions for the corresponding metal ions including the reduction of Fe2+. J2+ + 2e- → J Eo = - 1.66 v Fe2+ + 2e- → Fe Eo = - 0.44 v Q2+ + 2e- → Q Eo = - 0.25 v O2 + 2H2O + 4e- → 4OH- Eo = + 0.40 v
A group of students were tasked to perform the metal coupling of iron nail similar to Experiment 3: Corrosion of Metals. They were given two metals, metal Q and metal J, to couple to iron nail. They prepared an agar medium with K3Fe(CN)6 and phenolphthalein. Their professor informed them that metal Q forms an orange color while metal J forms maroon color when oxidized. Their professors also gave them the reduction half-reactions for the corresponding metal ions including the reduction of Fe2+.
J2+ + 2e- → J Eo = - 1.66 v
Fe2+ + 2e- → Fe Eo = - 0.44 v
Q2+ + 2e- → Q Eo = - 0.25 v
O2 + 2H2O + 4e- → 4OH- Eo = + 0.40 v
Step by step
Solved in 2 steps with 2 images









