A group of students were trying to experimentally determine the density of an unknown liquid. They developed a standard technique for measuring density by recording the mass of a liquid using an analytical balance. The volume of the liquids were accurately measured using a serological pipette and was recorded to be 5.00 mL for each trial. The students were able to collect the following data: Trial 1 (mass in grams) LIQUID Trial 2 (mass in Trial 3 (mass in Theoretical SAMPLE grams) grams) Density Hexane 3.281 3.284 3.279 0.655 g/mL Acetone 3.921 3.923 3.947 0.784 g/mL Ethanol 3.912 3.911 3.913 0.789 g/ml unknown 3.914 3.911 3.910
A group of students were trying to experimentally determine the density of an unknown liquid. They developed a standard technique for measuring density by recording the mass of a liquid using an analytical balance. The volume of the liquids were accurately measured using a serological pipette and was recorded to be 5.00 mL for each trial. The students were able to collect the following data: Trial 1 (mass in grams) LIQUID Trial 2 (mass in Trial 3 (mass in Theoretical SAMPLE grams) grams) Density Hexane 3.281 3.284 3.279 0.655 g/mL Acetone 3.921 3.923 3.947 0.784 g/mL Ethanol 3.912 3.911 3.913 0.789 g/ml unknown 3.914 3.911 3.910
Chapter14: Chromatography
Section: Chapter Questions
Problem 9P
Related questions
Question
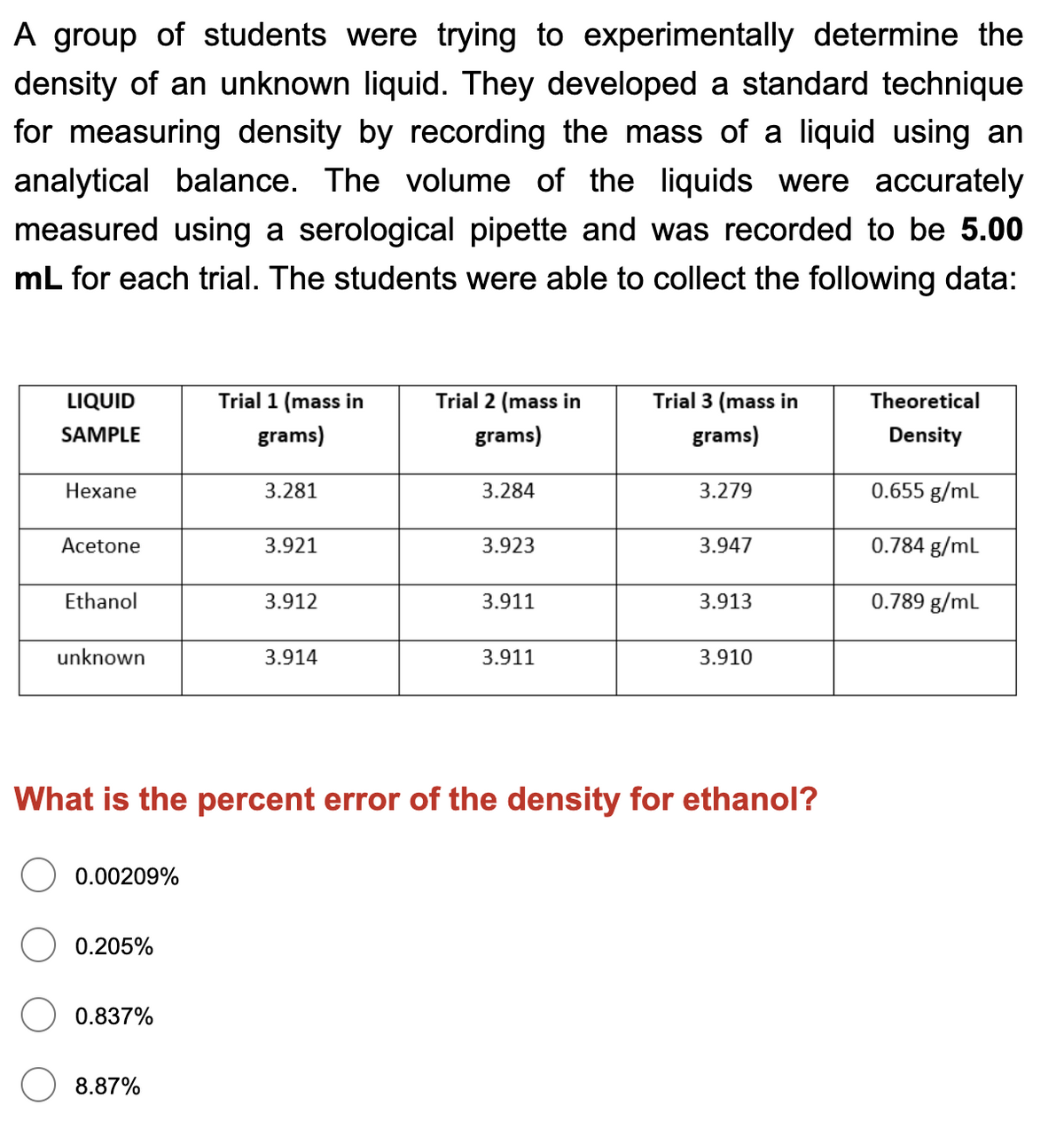
Transcribed Image Text:A group of students were trying to experimentally determine the
density of an unknown liquid. They developed a standard technique
for measuring density by recording the mass of a liquid using an
analytical balance. The volume of the liquids were accurately
measured using a serological pipette and was recorded to be 5.00
mL for each trial. The students were able to collect the following data:
LIQUID
Trial 1 (mass in
Trial 2 (mass in
Trial 3 (mass in
Theoretical
SAMPLE
grams)
grams)
grams)
Density
Hexane
3.281
3.284
3.279
0.655 g/ml
Acetone
3.921
3.923
3.947
0.784 g/ml
Ethanol
3.912
3.911
3.913
0.789 g/ml
unknown
3.914
3.911
3.910
What is the percent error of the density for ethanol?
0.00209%
0.205%
0.837%
8.87%
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



