Bobby Drake aka “Iceman” is member of the X-Men who has the physics-defying ability to “manipulate cold and ice.” Iceman is depicted with the ability to lower the temperature of nearby objects at will. For example, Iceman can freeze an entire pond in seconds just by touching the surface of the water. Let’s put some numbers to this example: The pond is 20 m across and can be approximated as a hemisphere. You can assume the density of water is 1.00 g cm–3 and independent of temperature. The temperature of the pond is initially 17°C and uniform throughout. After Iceman freezes the pond, its temperature is –10°C a) How many moles of water are in the pond? b) What is the total amount of heat removed from the pond?
Bobby Drake aka “Iceman” is member of the X-Men who has the physics-defying ability to “manipulate cold and ice.” Iceman is depicted with the ability to lower the temperature of nearby objects at will. For example, Iceman can freeze an entire pond in seconds just by touching the surface of the water. Let’s put some numbers to this example: The pond is 20 m across and can be approximated as a hemisphere. You can assume the density of water is 1.00 g cm–3 and independent of temperature. The temperature of the pond is initially 17°C and uniform throughout. After Iceman freezes the pond, its temperature is –10°C a) How many moles of water are in the pond? b) What is the total amount of heat removed from the pond?
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter6: Equilibria In Single-component Systems
Section: Chapter Questions
Problem 6.50E
Related questions
Question
Bobby Drake aka “Iceman” is member of the X-Men who has the physics-defying ability to “manipulate cold and ice.” Iceman is depicted with the ability to lower the temperature of nearby objects at will. For example, Iceman can freeze an entire pond in seconds just by touching the surface of the water. Let’s put some numbers to this example:
- The pond is 20 m across and can be approximated as a hemisphere.
- You can assume the density of water is 1.00 g cm–3 and independent of temperature.
- The temperature of the pond is initially 17°C and uniform throughout.
- After Iceman freezes the pond, its temperature is –10°C
a) How many moles of water are in the pond?
b) What is the total amount of heat removed from the pond?
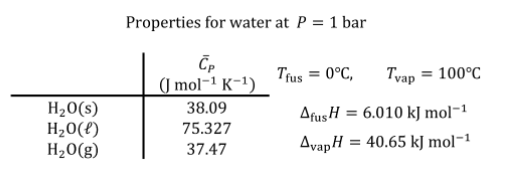
Transcribed Image Text:Properties for water at P = 1 bar
(J mol-1 K-1)
Trus = 0°C,
Tyap
= 100°C
H20(s)
H20(f)
H20(g)
38.09
AfusH
= 6.010 kJ mol-1
75.327
37.47
AvapH
= 40.65 kJ mol-1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,