A hot 102.4 g lump of an unknown substance initially at 168.9 °C is placed in 35.0 mL of water initially at 25.0 °C and Substance Specific heat (J/(g:°C)) aluminum 0.897 the system is allowed to reach thermal equilibrium. The final temperature of the system is 55.7 °C. graphite 0.709 rhodium 0.243 titanium 0.523 Using this information and the specific heat values for tungsten 0.132 zinc 0.388 several metals in the table, identify the unknown water 4.184 substance. Assume no heat is lost to the surroundings. O tungsten graphite O rhodium titanium O aluminum O zinc
A hot 102.4 g lump of an unknown substance initially at 168.9 °C is placed in 35.0 mL of water initially at 25.0 °C and Substance Specific heat (J/(g:°C)) aluminum 0.897 the system is allowed to reach thermal equilibrium. The final temperature of the system is 55.7 °C. graphite 0.709 rhodium 0.243 titanium 0.523 Using this information and the specific heat values for tungsten 0.132 zinc 0.388 several metals in the table, identify the unknown water 4.184 substance. Assume no heat is lost to the surroundings. O tungsten graphite O rhodium titanium O aluminum O zinc
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter4: Energy And Chemical Reactions
Section4.4: Heat Capacity
Problem 4.3PSP: A piece of aluminum with a mass of 250. g is at an initial temperature of 5.0 C. If 24.1 kJ is...
Related questions
Question
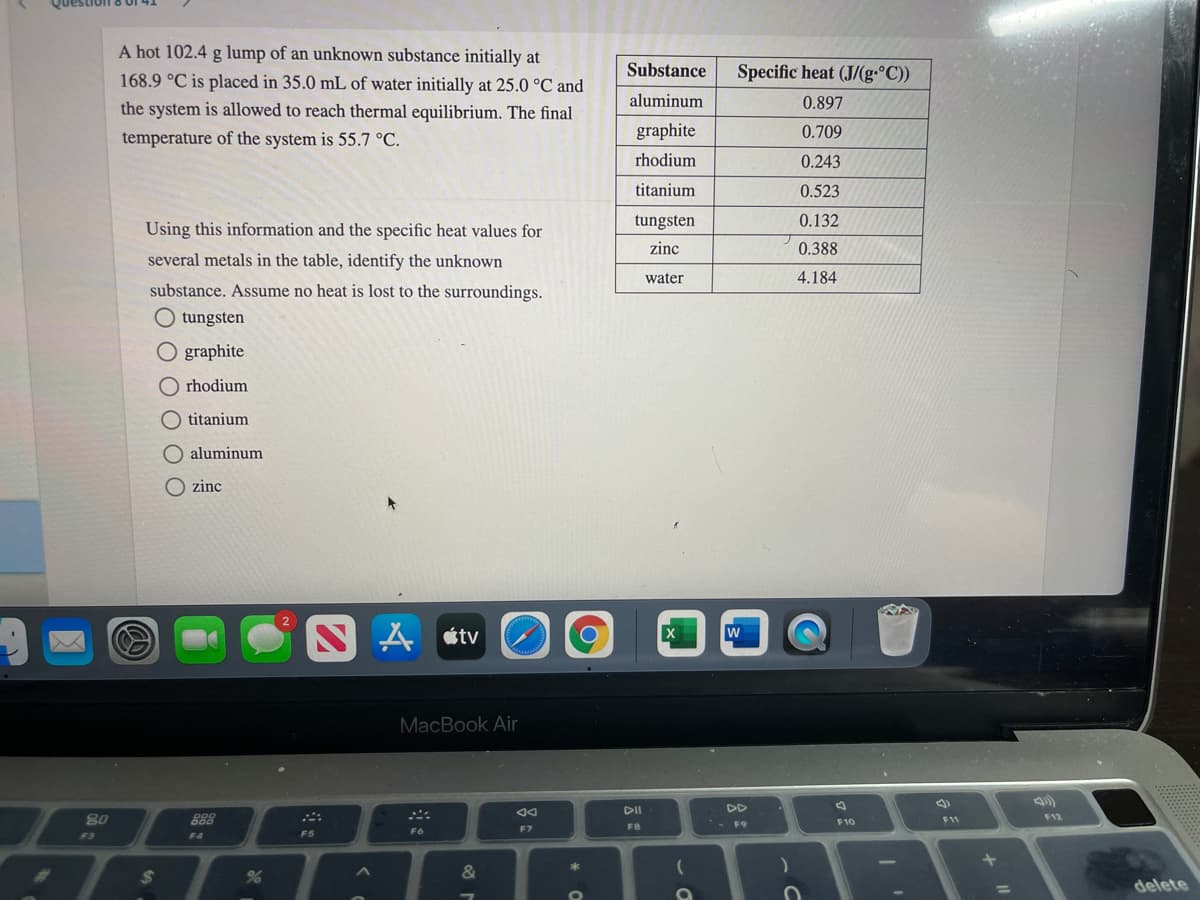
Transcribed Image Text:A hot 102.4 g lump of an unknown substance initially at
168.9 °C is placed in 35.0 mL of water initially at 25.0 °C and
Substance
Specific heat (J/(g-°C))
aluminum
0.897
the system is allowed to reach thermal equilibrium. The final
temperature of the system is 55.7 °C.
graphite
0.709
rhodium
0.243
titanium
0.523
tungsten
0.132
Using this information and the specific heat values for
zinc
0.388
several metals in the table, identify the unknown
water
4.184
substance. Assume no heat is lost to the surroundings.
O tungsten
O graphite
rhodium
titanium
O aluminum
O zinc
étv
W
MacBook Air
DII
80
888
F10
F11
F12
FB
F9
F6
F7
F3
F4
F5
24
%3D
delete
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning