A hydrogen atom is heated up causing an electron to be excited to a level which we will call E2. When the electron falls back to the E1 energy level, it emits a photon of light. The photon has an energy of 267.039 fJ. What is the energy change (∆E) of the electron when it falls from E2 to E1?
A hydrogen atom is heated up causing an electron to be excited to a level which we will call E2. When the electron falls back to the E1 energy level, it emits a photon of light. The photon has an energy of 267.039 fJ. What is the energy change (∆E) of the electron when it falls from E2 to E1?
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Chapter12: Unsaturated Hydrocarbons
Section: Chapter Questions
Problem 12.7E
Related questions
Question
A hydrogen atom is heated up causing an electron to be excited to a level which we will call E2. When the electron falls back to the E1 energy level, it emits a photon of light. The photon has an energy of 267.039 fJ. What is the energy change (∆E) of the electron when it falls from E2 to E1?
Enter your answer just as a number with units of fJ.
∆ is final minus initial.
Recall from thermo that the total energy change will be zero. (Light always has a positive energy).
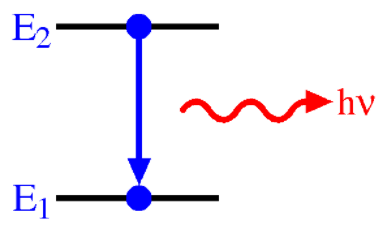
Transcribed Image Text:E2
hv
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning