A hydrogen gas is contained in a cylinder with a volume of 2.50 × 10¯³ m³ at atmospheric pressure. The gas and cylinder are at a thermal equilibrium of 30.0 °C. Assume that the hydrogen gas obeys the behaviors of monatomic ideal gas and the molar mass of hydrogen, Mmolar (H2) is 2.00 g mol¯!. (a) Calculate the number of moles of the hydrogen gas in the cylinder. Show that the r.m.s. speed of an ideal gas with a molar mass, Mmolar and at temperature T, can be given by (b) 3RT V rms M molar where R is the universal gas constant. Use the equation obtained in Q3. (b) to find the molecular r.m.s. speed of the hydrogen gas. (c)
A hydrogen gas is contained in a cylinder with a volume of 2.50 × 10¯³ m³ at atmospheric pressure. The gas and cylinder are at a thermal equilibrium of 30.0 °C. Assume that the hydrogen gas obeys the behaviors of monatomic ideal gas and the molar mass of hydrogen, Mmolar (H2) is 2.00 g mol¯!. (a) Calculate the number of moles of the hydrogen gas in the cylinder. Show that the r.m.s. speed of an ideal gas with a molar mass, Mmolar and at temperature T, can be given by (b) 3RT V rms M molar where R is the universal gas constant. Use the equation obtained in Q3. (b) to find the molecular r.m.s. speed of the hydrogen gas. (c)
Elements Of Electromagnetics
7th Edition
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Sadiku, Matthew N. O.
ChapterMA: Math Assessment
Section: Chapter Questions
Problem 1.1MA
Related questions
Question
PLEASE HELP ANSWER THIS
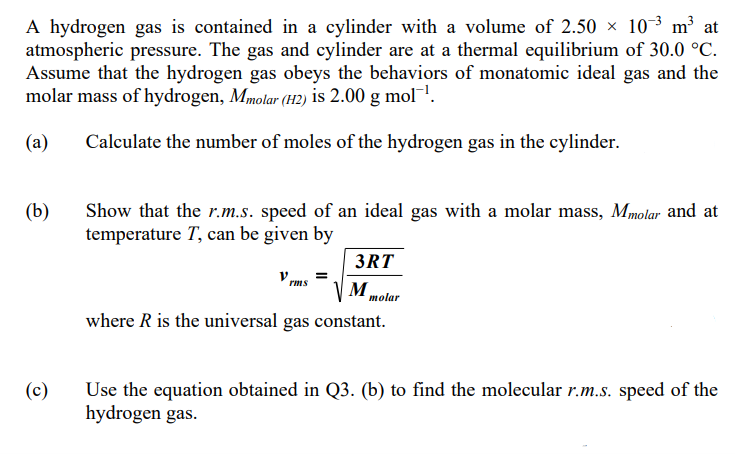
Transcribed Image Text:A hydrogen gas is contained in a cylinder with a volume of 2.50 × 10³ m³ at
atmospheric pressure. The gas and cylinder are at a thermal equilibrium of 30.0 °C.
Assume that the hydrogen gas obeys the behaviors of monatomic ideal gas and the
molar mass of hydrogen, Mmolar (H2) is 2.00 g mol!.
(a)
Calculate the number of moles of the hydrogen gas in the cylinder.
(b)
Show that the r.m.s. speed of an ideal gas with a molar mass, Mmolar and at
temperature T, can be given by
3RT
V rms
=
M.
molar
where R is the universal gas constant.
(c)
Use the equation obtained in Q3. (b) to find the molecular r.m.s. speed of the
hydrogen gas.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:
9781118170519
Author:
Norman S. Nise
Publisher:
WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:
9781337093347
Author:
Barry J. Goodno, James M. Gere
Publisher:
Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:
9781118807330
Author:
James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:
WILEY