a) Is it possible to have a poly(methyl methacrylate) homopolymer with the following molecular weight data and a degree of polymerization of 527? Why or why not? Molar mass of CSH8O2 = 100.21 g/mol
a) Is it possible to have a poly(methyl methacrylate) homopolymer with the following molecular weight data and a degree of polymerization of 527? Why or why not? Molar mass of CSH8O2 = 100.21 g/mol
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter23: Polymeric Materials And Soft Condensed Matter
Section: Chapter Questions
Problem 24AP
Related questions
Question
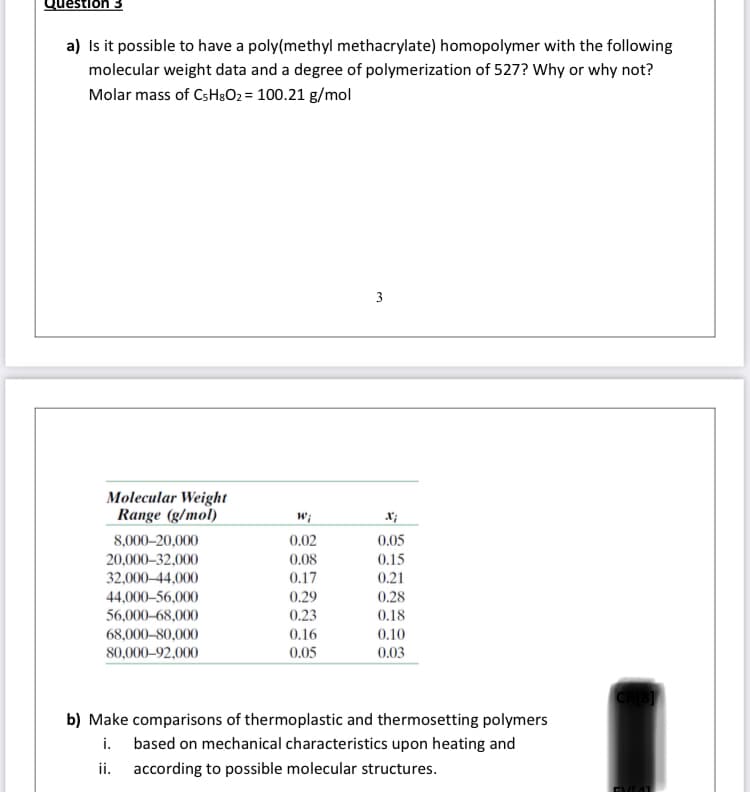
Transcribed Image Text:Question 3
a) Is it possible to have a poly(methyl methacrylate) homopolymer with the following
molecular weight data and a degree of polymerization of 527? Why or why not?
Molar mass of CsH3O2 = 100.21 g/mol
3
Molecular Weight
Range (g/mol)
w;
0.05
0.15
8,000–20,000
0.02
20,000–32,000
0.08
32,000–44,000
0.17
0.21
44,000–56,000
56,000-68,000
0.29
0.28
0.18
0.23
0.16
0.05
0.10
0.03
68,000-80,000
80,000–92,000
b) Make comparisons of thermoplastic and thermosetting polymers
i. based on mechanical characteristics upon heating and
i.
according to possible molecular structures.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning