A lab is performed by students who used diagnostic tests to try to distinguish among four pure substances. Solids labelled as 1, 2, 3, and 4 were dissolved in 100 ml of distilled water in four separate beakers with each becoming a colourless solution. The results of two diagnostic tests were recorded as follows: Solution Result of Conductivity Test Result of Litmus Test conducts no change 2 conducts blue turns red 3 does not conduct no change 4. conducts red turns blue Using the results of the diagnostic tests, select the identity for each solution. 1. Potassium bromide is the solution in beak 1 2. Phosphoric acid is the solution in beaker 2 3. Sodium hydroxide is the solution in beake 4. Sucrose is the solution in beaker
A lab is performed by students who used diagnostic tests to try to distinguish among four pure substances. Solids labelled as 1, 2, 3, and 4 were dissolved in 100 ml of distilled water in four separate beakers with each becoming a colourless solution. The results of two diagnostic tests were recorded as follows: Solution Result of Conductivity Test Result of Litmus Test conducts no change 2 conducts blue turns red 3 does not conduct no change 4. conducts red turns blue Using the results of the diagnostic tests, select the identity for each solution. 1. Potassium bromide is the solution in beak 1 2. Phosphoric acid is the solution in beaker 2 3. Sodium hydroxide is the solution in beake 4. Sucrose is the solution in beaker
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter15: Solutions
Section: Chapter Questions
Problem 23CR
Related questions
Question
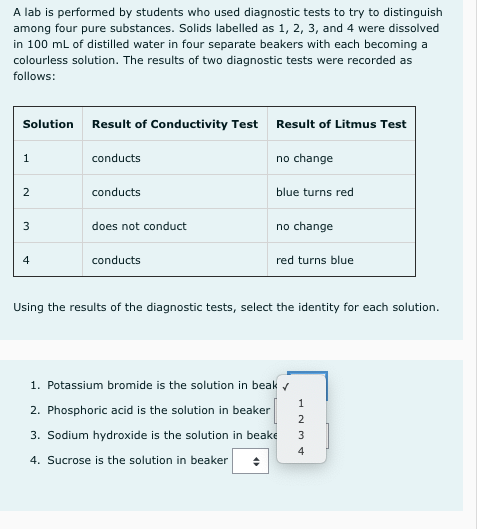
Transcribed Image Text:A lab is performed by students who used diagnostic tests to try to distinguish
among four pure substances. Solids labelled as 1, 2, 3, and 4 were dissolved
in 100 ml of distilled water in four separate beakers with each becoming a
colourless solution. The results of two diagnostic tests were recorded as
follows:
Solution
Result of Conductivity Test Result of Litmus Test
1
conducts
no change
2
conducts
blue turns red
3
does not conduct
no change
4
conducts
red turns blue
Using the results of the diagnostic tests, select the identity for each solution.
1. Potassium bromide is the solution in beak r
2. Phosphoric acid is the solution in beaker
3. Sodium hydroxide is the solution in beake
3
4
4. Sucrose is the solution in beaker
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning