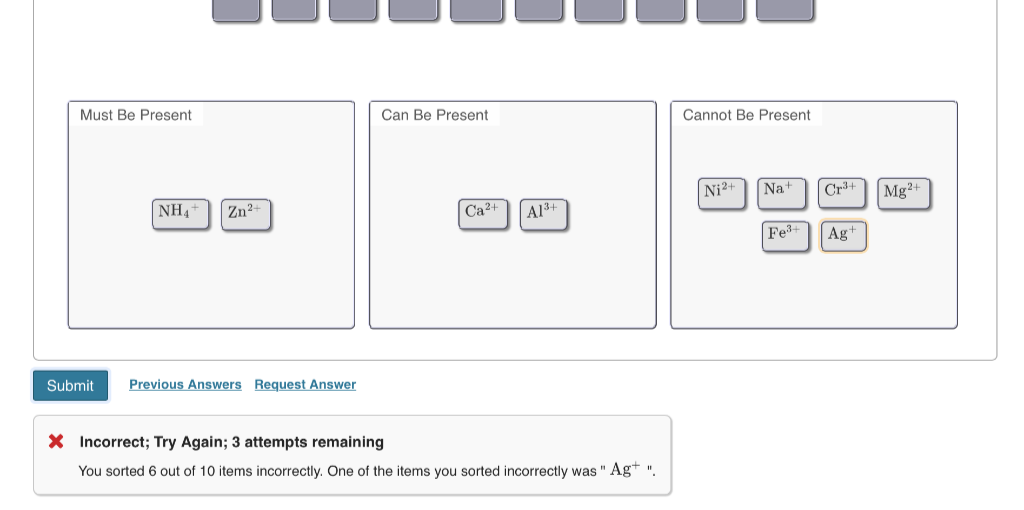
▼ Part A - Solution 1 After being given a solution containing a set of unknown ions, you perform the following sequence of tests to determine the identity of the cations in solution: 1. A flame test produces no color 2. You take a small portion of the solution and add NaOH. A vapor is produced that turns red litmus blue. 3. You add HCI to solution, which results in the formation of a white precipitate. The supernatant is separated and used for the remainder of the tests. 4. The addition of buffered NH4*/NH3 to a portion of the supernatant from step 3 does not produce a precipitate. 5. The addition of ammonium oxalate to a portion of the superntant from step 3 does not produce a precipitate 6. The addition of a strong base to a portion of the supernatant from step 3 generates a precipitate. 7. The supernatant from step 6 is separated from the precipitate. The addition of HCI and K4Fe(CN)6 to the solution produces no reaction. Based on these tests, identify which of the following ions must be present, can be present, and cannot be present. Na+, NH4+, Ag+, Fe³+, Al³+, Cr³+, Ca2+, Mg²+, Ni²+, Zn²+
Ionic Equilibrium
Chemical equilibrium and ionic equilibrium are two major concepts in chemistry. Ionic equilibrium deals with the equilibrium involved in an ionization process while chemical equilibrium deals with the equilibrium during a chemical change. Ionic equilibrium is established between the ions and unionized species in a system. Understanding the concept of ionic equilibrium is very important to answer the questions related to certain chemical reactions in chemistry.
Arrhenius Acid
Arrhenius acid act as a good electrolyte as it dissociates to its respective ions in the aqueous solutions. Keeping it similar to the general acid properties, Arrhenius acid also neutralizes bases and turns litmus paper into red.
Bronsted Lowry Base In Inorganic Chemistry
Bronsted-Lowry base in inorganic chemistry is any chemical substance that can accept a proton from the other chemical substance it is reacting with.

Trending now
This is a popular solution!
Step by step
Solved in 2 steps









