A layer of 10 g of iodine is spread uniformly over a surface of area 10 cm? and covered in hexane to a depth of 10 cm. Diffusion coefficient of I2 in hexane is 4.05 · 10-9 m2 What will be the molar concentration of I2 at 5 cm above the original layer after: a) 10 s?; b) 24 h?; c) compare the answer in b) with the maximum concentration achieved if I2 were completely dissolved in the cubic volume 10 cm² x 5 cm and based on this comparison conclude if the diffusion will continue beyond 24 h or not
A layer of 10 g of iodine is spread uniformly over a surface of area 10 cm? and covered in hexane to a depth of 10 cm. Diffusion coefficient of I2 in hexane is 4.05 · 10-9 m2 What will be the molar concentration of I2 at 5 cm above the original layer after: a) 10 s?; b) 24 h?; c) compare the answer in b) with the maximum concentration achieved if I2 were completely dissolved in the cubic volume 10 cm² x 5 cm and based on this comparison conclude if the diffusion will continue beyond 24 h or not
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter8: Properties Of Gases
Section8.7: Kinetic-molecular Theory And The Velocities Of Gas Molecules
Problem 8.17E
Related questions
Question
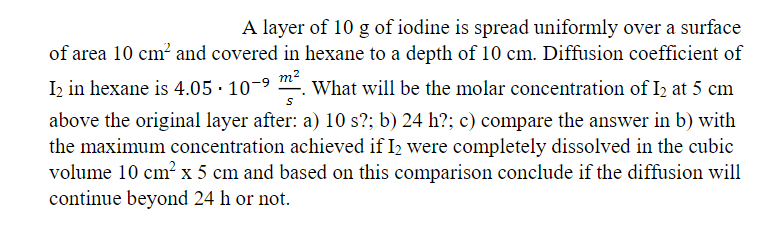
Transcribed Image Text:A layer of 10 g of iodine is spread uniformly over a surface
of area 10 cm? and covered in hexane to a depth of 10 cm. Diffusion coefficient of
I2 in hexane is 4.05 · 10-9
m2
What will be the molar concentration of I2 at 5 cm
above the original layer after: a) 10 s?; b) 24 h?; c) compare the answer in b) with
the maximum concentration achieved if I2 were completely dissolved in the cubic
volume 10 cm² x 5 cm and based on this comparison conclude if the diffusion will
continue beyond 24 h or not
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning
