A mass of 31.5 g of H20(g) at 373 Kis allowed to flow into 279 g of H2O(1) at 298 K and 1 atm Part A Cem for H,O is constant at its values for 298 K (75.3 J. mol 1. K 1) throughout the temperature range of interest AHan for water is 40650 Calculate the final temperature of the system once equilibrium has been reached. Assume J. mol 1 Express the temperature in kelvins to three significant figures. T = K Submit Reguest Answer
A mass of 31.5 g of H20(g) at 373 Kis allowed to flow into 279 g of H2O(1) at 298 K and 1 atm Part A Cem for H,O is constant at its values for 298 K (75.3 J. mol 1. K 1) throughout the temperature range of interest AHan for water is 40650 Calculate the final temperature of the system once equilibrium has been reached. Assume J. mol 1 Express the temperature in kelvins to three significant figures. T = K Submit Reguest Answer
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter2: The First Law Of Thermodynamics
Section: Chapter Questions
Problem 2.32E: Many compressed gases come in large,heavy metal cylindersthat are so heavy that they need a special...
Related questions
Question
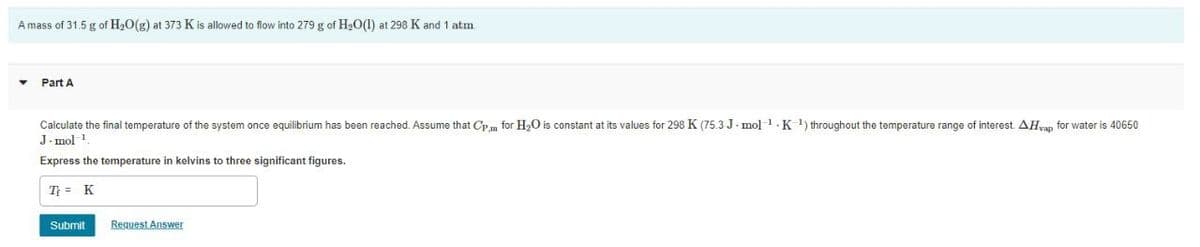
Transcribed Image Text:A mass of 31.5 g of H20(g) at 373 K is allowed to flow into 279 g of H20(1) at 298 K and 1 atm
Part A
Calculate the final temperature of the system once equilibrium has been reached. Assume that Cp m for H,O is constant at its values for 298 K (75.3 J. mol1.K 1) throughout the temperature range of interest AHyan for water is 40650
J. mol 1
Express the temperature in kelvins to three significant figures.
T = K
Submit
Request Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning