A mineral sample is obtained from a region of the country that has high arsenic contamination. An elemental analysis yields the following elemental composition: Element Atomic Weight (g/mol) Percent Composition Fe 55.845 74.9216 20.1% 53.9% As ||15.9994 25.9% 0.181% H 1.00794 What is the empirical formula of this mineral?
A mineral sample is obtained from a region of the country that has high arsenic contamination. An elemental analysis yields the following elemental composition: Element Atomic Weight (g/mol) Percent Composition Fe 55.845 74.9216 20.1% 53.9% As ||15.9994 25.9% 0.181% H 1.00794 What is the empirical formula of this mineral?
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter2: Chemical Formulas, Equations, And Reaction Yields
Section: Chapter Questions
Problem 9P: Fulgurites are the products of the melting that occurs when lightning strikes the earth. Microscopic...
Related questions
Question
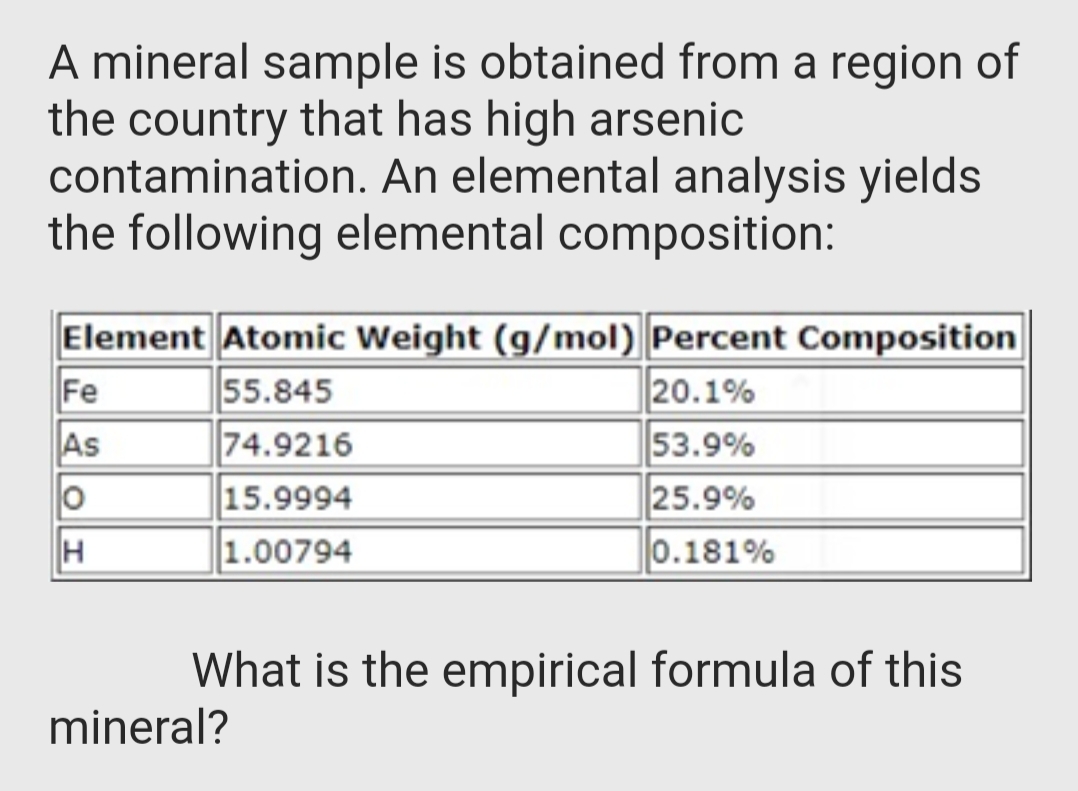
Transcribed Image Text:A mineral sample is obtained from a region of
the country that has high arsenic
contamination. An elemental analysis yields
the following elemental composition:
Element Atomic Weight (g/mol) Percent Composition
Fe
55.845
74.9216
15.9994
20.1%
53.9%
25.9%
0.181%
As
H
1.00794
What is the empirical formula of this
mineral?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
