To help farmers and gardeners, commercial fertilizers have a big three-number "NPK" label on the bag that gives the amounts of three key plant nutrients: nitrogen, phosphorus and potassium. For example, a bag of "10- 10- 10" fertilizer has 10% by mass nitrogen, 10% by mass potassium as potash (K,0), and 10% by mass phosphorus as phosphate (P205). Suppose a certain fertilizer has the following composition: ingredient percent by mass ammonium phosphate ((NH,), PO,) 37% (NH.),CO) urea 40% potassium chloride (KCI) 8% Inert ingredients 15% What should the first (nitrogen) number on the bag label be? That is, calculate the percent by mass of nitrogen in this fertilizer. Round your answer to the nearest percent. 山回
To help farmers and gardeners, commercial fertilizers have a big three-number "NPK" label on the bag that gives the amounts of three key plant nutrients: nitrogen, phosphorus and potassium. For example, a bag of "10- 10- 10" fertilizer has 10% by mass nitrogen, 10% by mass potassium as potash (K,0), and 10% by mass phosphorus as phosphate (P205). Suppose a certain fertilizer has the following composition: ingredient percent by mass ammonium phosphate ((NH,), PO,) 37% (NH.),CO) urea 40% potassium chloride (KCI) 8% Inert ingredients 15% What should the first (nitrogen) number on the bag label be? That is, calculate the percent by mass of nitrogen in this fertilizer. Round your answer to the nearest percent. 山回
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter14: Mass Spectrometry
Section: Chapter Questions
Problem 14.38P
Related questions
Question
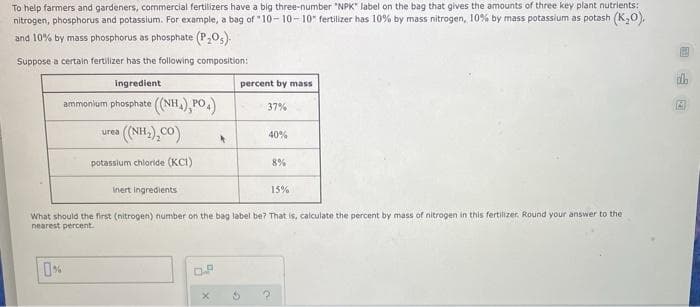
Transcribed Image Text:To help farmers and gardeners, commercial fertilizers have a big three-number "NPK" label on the bag that gives the amounts of three key plant nutrients:
nitrogen, phosphorus and potassium. For example, a bag of "10- 10-10" fertilizer has 10% by mass nitrogen, 10% by mass potassium as potash (K,0),
and 10% by mass phosphorus as phosphate (P205).
Suppose a certain fertilizer has the following composition:
ingredient
percent by mass
ammonium phosphate ((NH,) PO,)
37%
(NH.),CO)
urea
40%
potassium chloride (KCI)
8%
Inert ingredients
15%
What should the first (nitrogen) number on the bag label be? That is, calculate the percent by mass of nitrogen in this fertilizer. Round your answer to the
nearest percent.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning