A mixture of gaseous reactants is put into a cylinder, where a chemical reaction turns them into gaseous products. The 1 atm pressure cylinder has a piston that moves in or out, as necessary, to keep a constant pressure on the mixture of 1 atm. The cylinder is also submerged in a large insulated water bath. (See sketch at right.) piston cylinder The temperature of the water bath is monitored, and it is determined from this data that 338. kJ of heat flows out of the system during the reaction. The position of the piston is also monitored, and it is determined from this data that the piston does 117. kJ of work on the system during the reaction. water bath gases exothermic Is the reaction exothermic or endothermic? endothermic up Does the temperature of the water bath go up or down? down neither in out Does the piston move in or out? neither absorb release Does the reaction absorb or release energy? neither ? X Submit Assignment Terms of Use Privacy 2019 McGraw-Hill Education. All Rights Reserved. MacBook Pro DD DII OO0 000 SC F10 F9 F 5 F8 F 6 F7 F 4 F3 FT
A mixture of gaseous reactants is put into a cylinder, where a chemical reaction turns them into gaseous products. The 1 atm pressure cylinder has a piston that moves in or out, as necessary, to keep a constant pressure on the mixture of 1 atm. The cylinder is also submerged in a large insulated water bath. (See sketch at right.) piston cylinder The temperature of the water bath is monitored, and it is determined from this data that 338. kJ of heat flows out of the system during the reaction. The position of the piston is also monitored, and it is determined from this data that the piston does 117. kJ of work on the system during the reaction. water bath gases exothermic Is the reaction exothermic or endothermic? endothermic up Does the temperature of the water bath go up or down? down neither in out Does the piston move in or out? neither absorb release Does the reaction absorb or release energy? neither ? X Submit Assignment Terms of Use Privacy 2019 McGraw-Hill Education. All Rights Reserved. MacBook Pro DD DII OO0 000 SC F10 F9 F 5 F8 F 6 F7 F 4 F3 FT
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter12: Thermodynamic Processes And Thermochemistry
Section: Chapter Questions
Problem 81AP
Related questions
Question
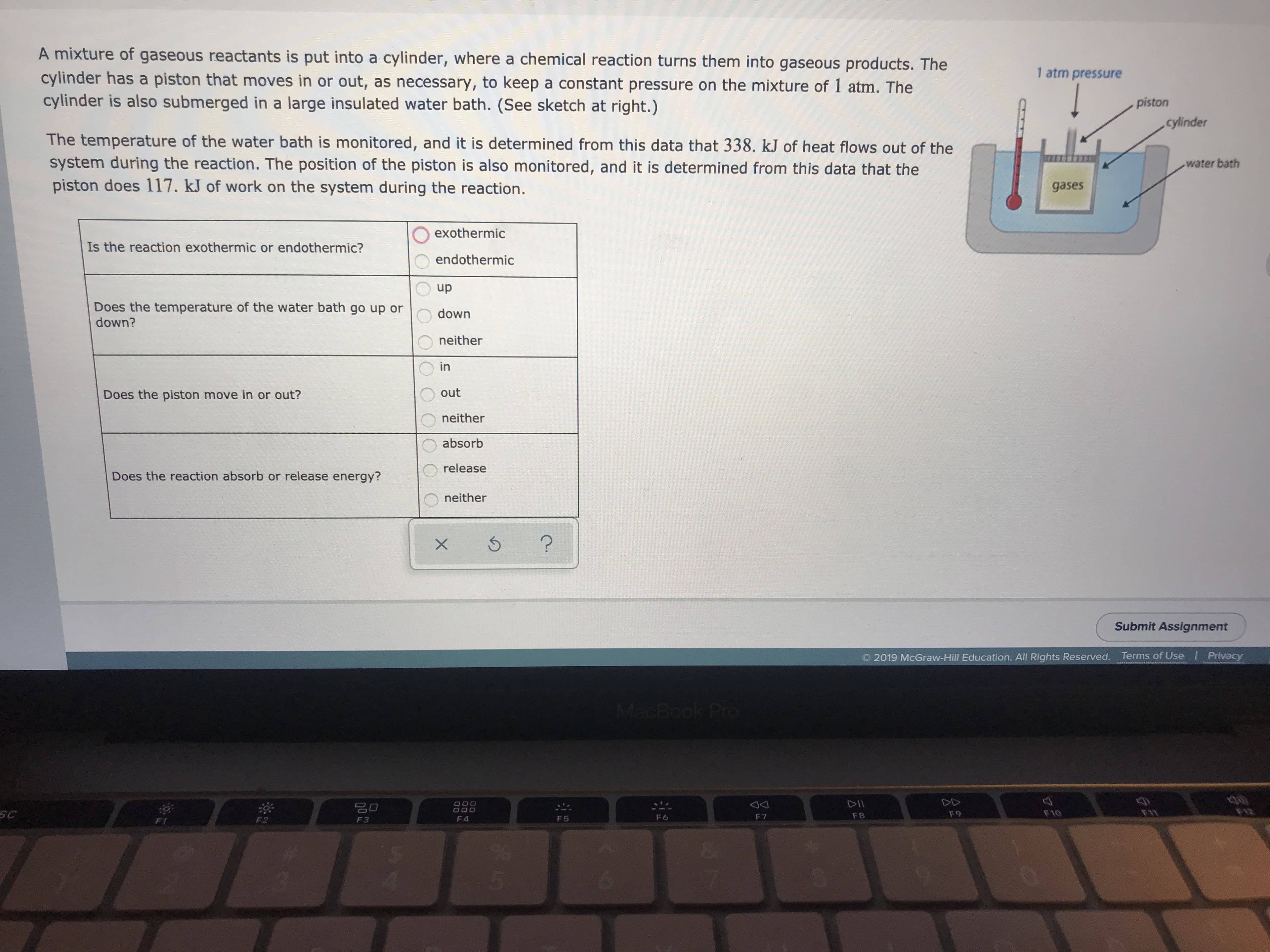
Transcribed Image Text:A mixture of gaseous reactants is put into a cylinder, where a chemical reaction turns them into gaseous products. The
1 atm pressure
cylinder has a piston that moves in or out, as necessary, to keep a constant pressure on the mixture of 1 atm. The
cylinder is also submerged in a large insulated water bath. (See sketch at right.)
piston
cylinder
The temperature of the water bath is monitored, and it is determined from this data that 338. kJ of heat flows out of the
system during the reaction. The position of the piston is also monitored, and it is determined from this data that the
piston does 117. kJ of work on the system during the reaction.
water bath
gases
exothermic
Is the reaction exothermic or endothermic?
endothermic
up
Does the temperature of the water bath go up or
down?
down
neither
in
out
Does the piston move in or out?
neither
absorb
release
Does the reaction absorb or release energy?
neither
?
X
Submit Assignment
Terms of Use
Privacy
2019 McGraw-Hill Education. All Rights Reserved.
MacBook Pro
DD
DII
OO0
000
SC
F10
F9
F 5
F8
F 6
F7
F 4
F3
FT
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning