A new linear temperature scale, degree compound zunzol. The freezing point of zunzol, –-141.5 °C, is defined as 0 °Z, and the boiling point of zunzol, -24.8 °C, is defined as 100 °Z. What is the freezing point of water in degrees Zunzol? freezing point: What is the boiling point of water in degrees Zunzol? boiling point:
A new linear temperature scale, degree compound zunzol. The freezing point of zunzol, –-141.5 °C, is defined as 0 °Z, and the boiling point of zunzol, -24.8 °C, is defined as 100 °Z. What is the freezing point of water in degrees Zunzol? freezing point: What is the boiling point of water in degrees Zunzol? boiling point:
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter5: Principles Of Chemical Reactivity: Energy And Chemical Reactions
Section: Chapter Questions
Problem 106SCQ: You are attending summer school and living in a very old dormitory. The day is oppressively hot....
Related questions
Question
Transcribed Image Text:SylvanPrep
edx edX| Free Online...
C Coursera| Online...
= College Algebra B..
an...
- Calcworkshop
Resources
O Hint
Check Answer
45.9%
Assignment Score:
Question 32 of 41
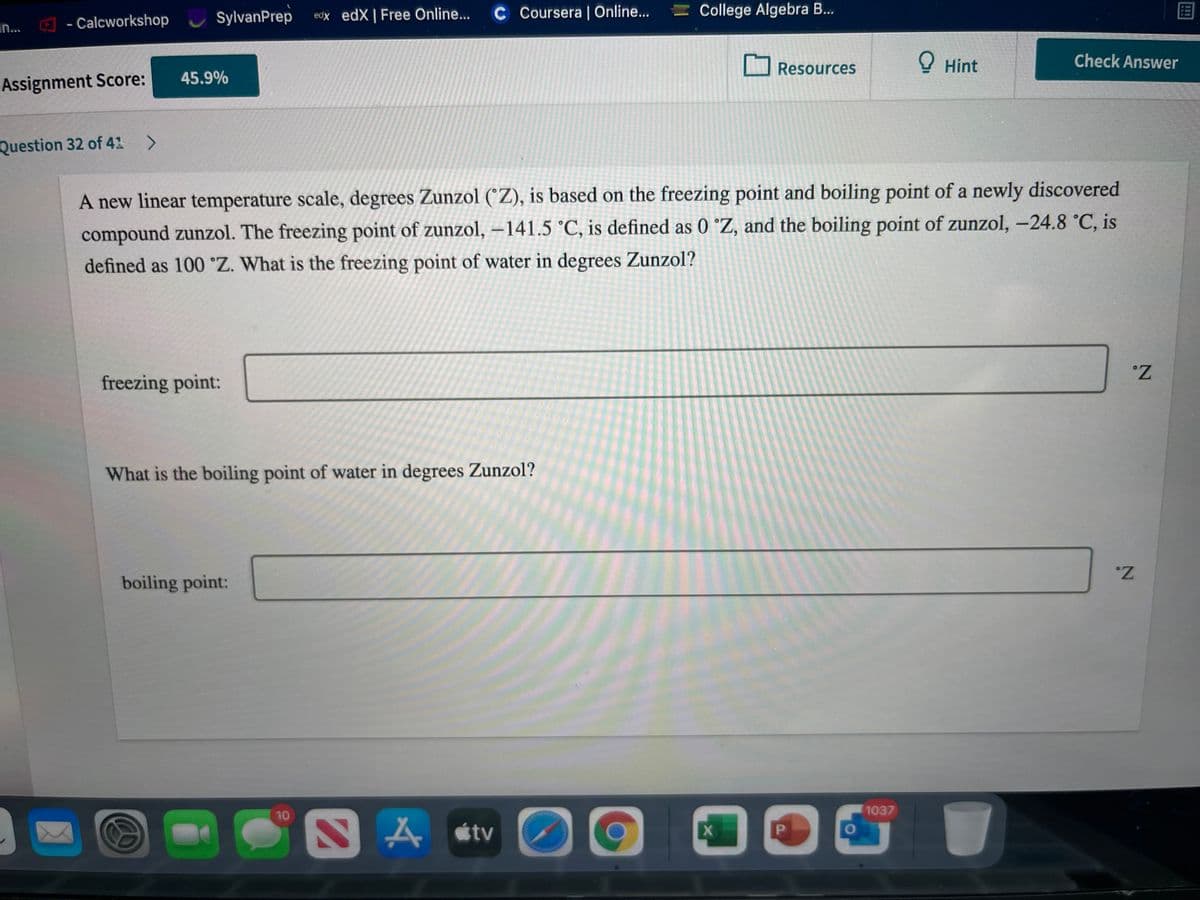
A new linear temperature scale, degrees Zunzol (°Z), is based on the freezing point and boiling point of a newly discovered
compound zunzol. The freezing point of zunzol, -141.5 °C, is defined as 0 °Z, and the boiling point of zunzol, -24.8 °C, is
defined as 100 °Z. What is the freezing point of water in degrees Zunzol?
|
freezing point:
What is the boiling point of water in degrees Zunzol?
boiling point:
1037
10
A stv
P.
2.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning