A particular organic molecule has a weight of 92 and provides the given mass spectrum below. a. Draw the structure of the molecule that matches the graph below. b. Demonstrate the electron-pushing arrows that account for the given fragmentation masses
A particular organic molecule has a weight of 92 and provides the given mass spectrum below. a. Draw the structure of the molecule that matches the graph below. b. Demonstrate the electron-pushing arrows that account for the given fragmentation masses
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter31: Thermal Methods
Section: Chapter Questions
Problem 31.11QAP: Describe the difference between power-compensated, heat-flux, and modulated DSC instruments.
Related questions
Question
Chemistry
I need help with a homework problem. A particular organic molecule has a weight of 92 and provides the given mass spectrum below.
a. Draw the structure of the molecule that matches the graph below.
b. Demonstrate the electron-pushing arrows that account for the given fragmentation masses.
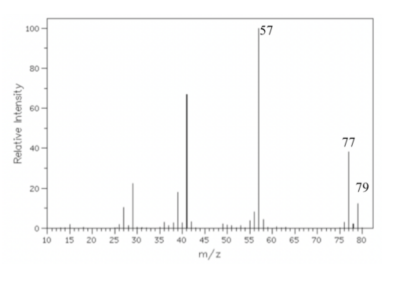
Transcribed Image Text:Relative Intensity
100
80
40
20-
157
77
79
oftm
10 15 20 25 30 35 40 45 50 55 60 65 70 75 80
m/z
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning