From mass to moles There are two unknowns in this problem - the grams of potassium carbonates and the grams of sodium carbonate (we only know the combined grams). Let's designate the grams of potassium carbonate as our first unknown (you may want to call it gKcarb, or x, some other variable name that makes sense to you) and the grams of sodium carbonate as our second unknown (you may want to call it gNacarb, or y, some other variable name that makes sense to you). Set up an equation for the sum of your two unknowns. Starting with 'unknown' grams of potassium carbonate, use stoichiometry to calculate the number of moles of nitric acid that would react with the potassium carbonate. Your answer will have a variable for your unknown grams of potassium carbonate in it. Starting with 'unknown' grams of sodium carbonate, use stoichiometry to calculate the number of moles of nitric acid that would react with the sodium carbonate. Your answer will have a variable for your unknown grams of sodium carbonate in it. Set up an equation for what you get if you add these two quantities. You should now have two equations (sum of your masses, and sum of moles of acid) and two unknowns! solve your system of equations for the mass of potassium carbonate and the mass of sodium carbonate. mass potassium carbonate (x) g mass sodium carbonate (y) g Evaluate
From mass to moles There are two unknowns in this problem - the grams of potassium carbonates and the grams of sodium carbonate (we only know the combined grams). Let's designate the grams of potassium carbonate as our first unknown (you may want to call it gKcarb, or x, some other variable name that makes sense to you) and the grams of sodium carbonate as our second unknown (you may want to call it gNacarb, or y, some other variable name that makes sense to you). Set up an equation for the sum of your two unknowns. Starting with 'unknown' grams of potassium carbonate, use stoichiometry to calculate the number of moles of nitric acid that would react with the potassium carbonate. Your answer will have a variable for your unknown grams of potassium carbonate in it. Starting with 'unknown' grams of sodium carbonate, use stoichiometry to calculate the number of moles of nitric acid that would react with the sodium carbonate. Your answer will have a variable for your unknown grams of sodium carbonate in it. Set up an equation for what you get if you add these two quantities. You should now have two equations (sum of your masses, and sum of moles of acid) and two unknowns! solve your system of equations for the mass of potassium carbonate and the mass of sodium carbonate. mass potassium carbonate (x) g mass sodium carbonate (y) g Evaluate
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter3: Calculations With Chemical Formulas And Equaitons
Section: Chapter Questions
Problem 3.17QP
Related questions
Question
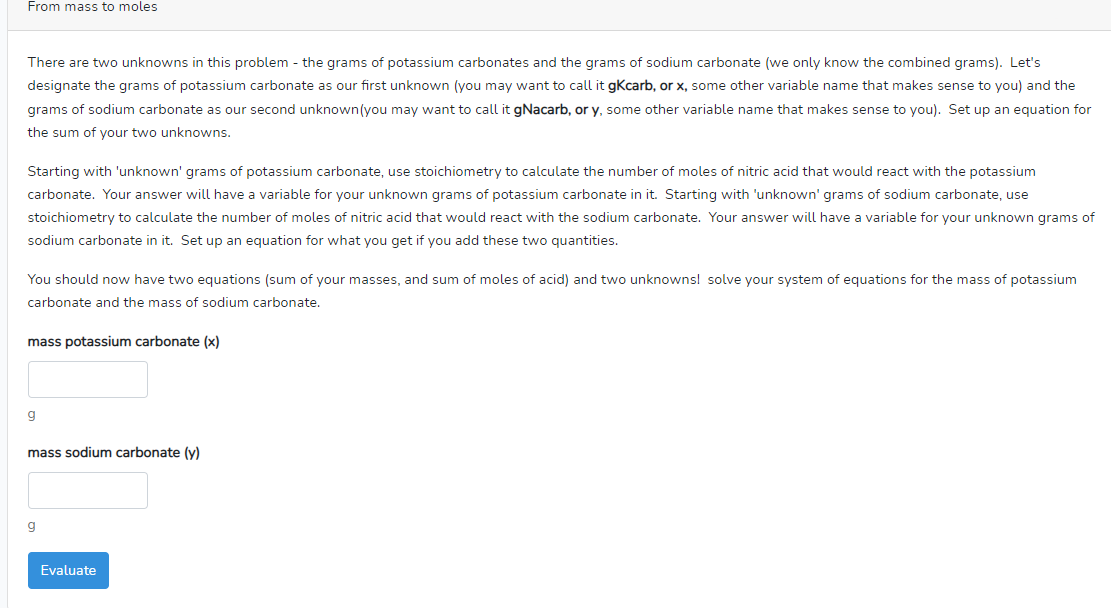
Transcribed Image Text:From mass to moles
There are two unknowns in this problem - the grams of potassium carbonates and the grams of sodium carbonate (we only know the combined grams). Let's
designate the grams of potassium carbonate as our first unknown (you may want to call it gKcarb, or x, some other variable name that makes sense to you) and the
grams of sodium carbonate as our second unknown (you may want to call it gNacarb, or y, some other variable name that makes sense to you). Set up an equation for
the sum of your two unknowns.
Starting with 'unknown' grams of potassium carbonate, use stoichiometry to calculate the number of moles of nitric acid that would react with the potassium
carbonate. Your answer will have a variable for your unknown grams of potassium carbonate in it. Starting with 'unknown' grams of sodium carbonate, use
stoichiometry to calculate the number of moles of nitric acid that would react with the sodium carbonate. Your answer will have a variable for your unknown grams of
sodium carbonate in it. Set up an equation for what you get if you add these two quantities.
You should now have two equations (sum of your masses, and sum of moles of acid) and two unknowns! solve your system of equations for the mass of potassium
carbonate and the mass of sodium carbonate.
mass potassium carbonate (x)
g
mass sodium carbonate (y)
g
Evaluate
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning