A previous Chem 100 student carried out the experiment you did by reacting 0.04 g of magnesium. The following data was collected in their lab notebook. The water temperature was 19.8°C. The barometer in the lab read 757.9 mm Hg Sample of 36.2 mL of gas was collected The vapor pressure of water at this temperature is 17.32 mm Hg. Calculate the student's experimental value of R. Report your answer to three places after the decimal.
A previous Chem 100 student carried out the experiment you did by reacting 0.04 g of magnesium. The following data was collected in their lab notebook. The water temperature was 19.8°C. The barometer in the lab read 757.9 mm Hg Sample of 36.2 mL of gas was collected The vapor pressure of water at this temperature is 17.32 mm Hg. Calculate the student's experimental value of R. Report your answer to three places after the decimal.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter10: Solids, Liquids, And Phase Transitions
Section: Chapter Questions
Problem 35P
Related questions
Question
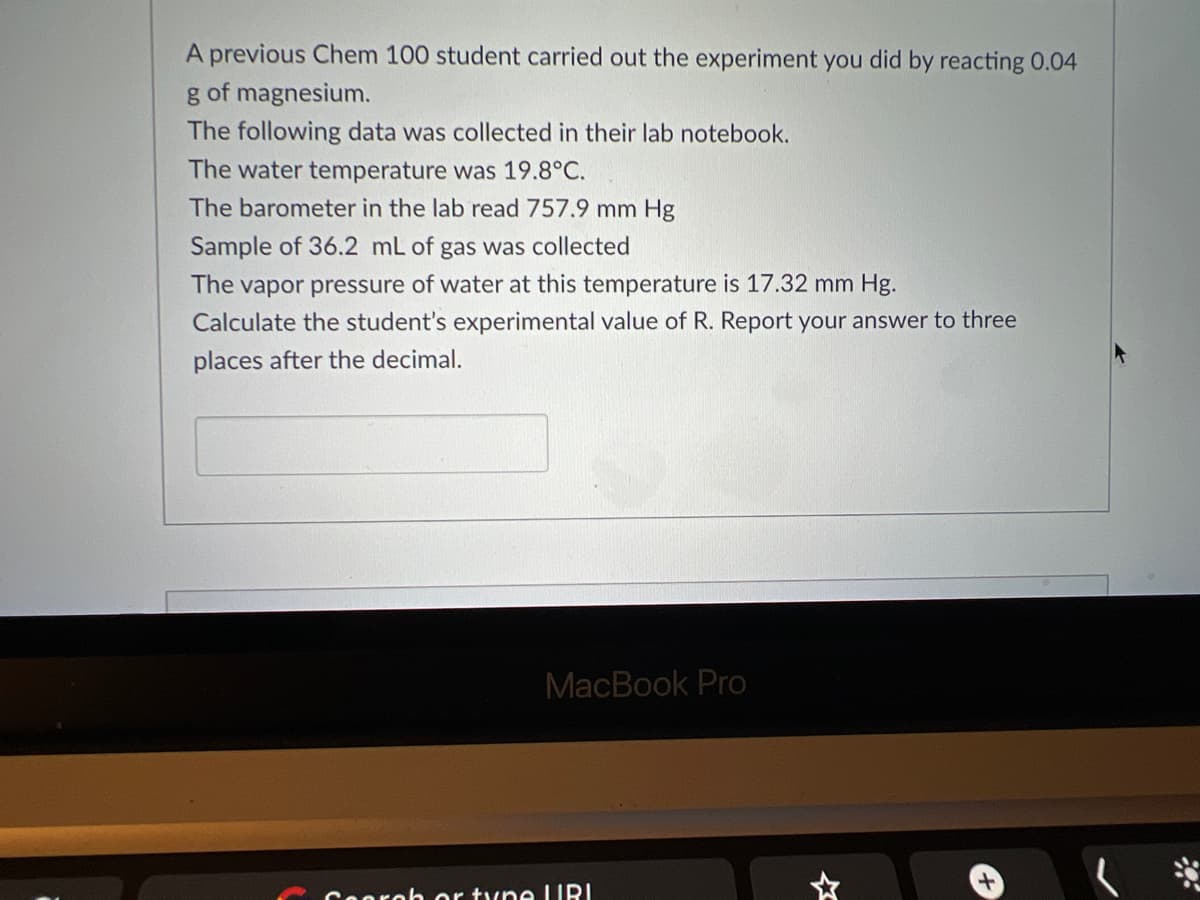
Transcribed Image Text:A previous Chem 100 student carried out the experiment you did by reacting 0.04
g of magnesium.
The following data was collected in their lab notebook.
The water temperature was 19.8°C.
The barometer in the lab read 757.9 mm Hg
Sample of 36.2 mL of gas was collected
The vapor pressure of water at this temperature is 17.32 mm Hg.
Calculate the student's experimental value of R. Report your answer to three
places after the decimal.
MacBook Pro
oh or tyne URI
+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning