A Question 10 (1 point) Retake question What is the percent by mass of 50 grams of NaCl dissolved in 50 grams of wate O1% O2 % O 100 % 50 % > View hint for Question 10 A Question 11 (1 point) kelke ueston How many Liters of solution do you need if you begin with 20 males of NaCl ar
A Question 10 (1 point) Retake question What is the percent by mass of 50 grams of NaCl dissolved in 50 grams of wate O1% O2 % O 100 % 50 % > View hint for Question 10 A Question 11 (1 point) kelke ueston How many Liters of solution do you need if you begin with 20 males of NaCl ar
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter10: Solutions
Section: Chapter Questions
Problem 71QAP: One mole of Na2S is represented as where represents Na and represents S. Complete the picture...
Related questions
Question
Im lost
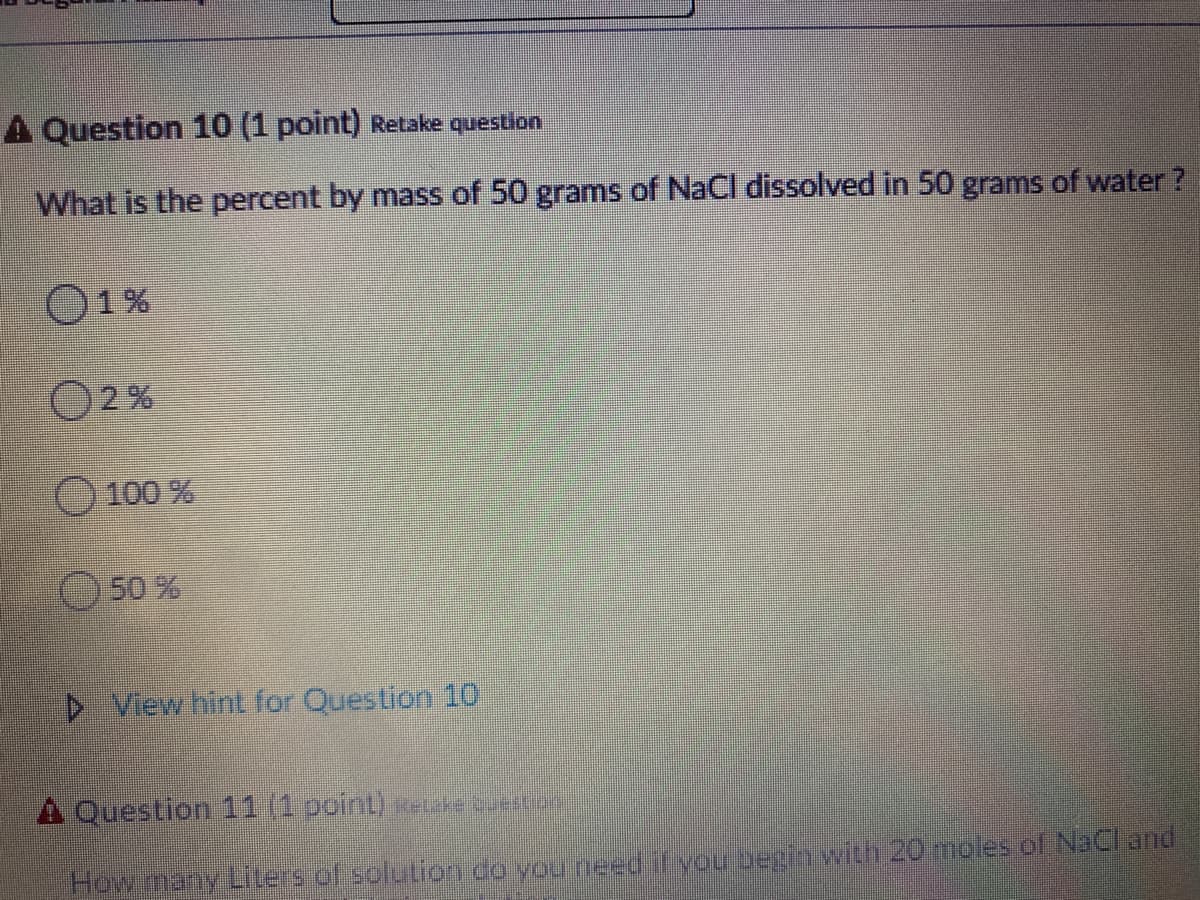
Transcribed Image Text:A Question 10 (1 point) Retake question
What is the percent by mass of 50 grams of NaCl dissolved in 50 grams of water?
O1%
O2 %
O 100 %
50 %
D View hint for Question 10
A Question 11 (1 point) keke uetor
How many Liters of solution do vou needif you beginwith 20 moles of NaCl and
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

