A researchers titrated a 100 mL solution of 0.1 M glycine at pH 1.72 with 2 M NaOH solution. She then monitored the pH and plotted the results in the graph shown here. The key points in the titration are designated I through V. 12 10 9.60 8 pH 6 4 11.30 2 5.97 2.34 (1) (II) 0.5 › (III) (IV) I 1.0 1.5 OH (equivalents) (V) 2.0
A researchers titrated a 100 mL solution of 0.1 M glycine at pH 1.72 with 2 M NaOH solution. She then monitored the pH and plotted the results in the graph shown here. The key points in the titration are designated I through V. 12 10 9.60 8 pH 6 4 11.30 2 5.97 2.34 (1) (II) 0.5 › (III) (IV) I 1.0 1.5 OH (equivalents) (V) 2.0
Chemical Principles in the Laboratory
11th Edition
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Chapter24: The Standardization Of A Basic Solution And The Determination Of The Molar Mass Of An Acid
Section: Chapter Questions
Problem 2ASA: In an acid-base titration, 21.16 mL of an NaOH solution are needed to neutralize 20.04 mL of a...
Related questions
Question
100%
For each of the statements, identify the appropriate key point(s) (I, II, III, IV, V) in the titration.
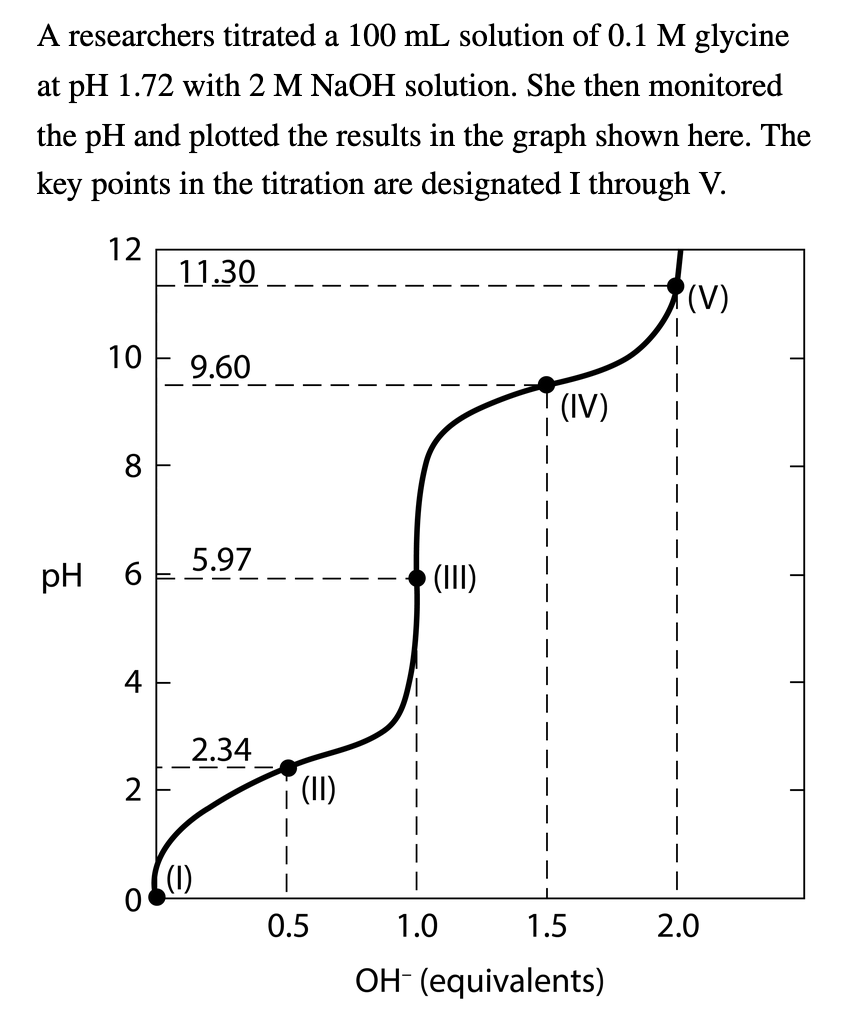
Transcribed Image Text:A
researchers titrated a 100 mL solution of 0.1 M glycine
at pH 1.72 with 2 M NaOH solution. She then monitored
the pH and plotted the results in the graph shown here. The
key points in the titration are designated I through V.
12
10
8
pH 6
4
2
11.30
9.60
5.97
2.34
(II)
I
0.5
(III)
(IV)
1.0
1.5
OH- (equivalents)
(V)
2.0

Transcribed Image Text:For each of the statements, identify the appropriate key
point(s) in the titration.
The average net charge of glycine is +1.
Half of the amino groups are ionized.
The average net charge of glycine is 0.
The average net charge of glycine is -1.
This is the isoelectric point for glycine.
Glycine has its maximum buffering capacity at
these points.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning