A sample consisting of Na2CO3 (105.96), NaOH (39.99) and inert matter weighs 1.179 g. It is titrated with 0.300 N HCI with phenolphthalein as the indicator, and the solution becomes colorless after the addition of 48.16 ml. Methyl orange is then added and 24.08 ml more of the acid are needed for the color change. What is the percentage of NaOH and N22CO3? *
A sample consisting of Na2CO3 (105.96), NaOH (39.99) and inert matter weighs 1.179 g. It is titrated with 0.300 N HCI with phenolphthalein as the indicator, and the solution becomes colorless after the addition of 48.16 ml. Methyl orange is then added and 24.08 ml more of the acid are needed for the color change. What is the percentage of NaOH and N22CO3? *
Chapter16: Applications Of Neutralization Titrations
Section: Chapter Questions
Problem 16.40QAP
Related questions
Question
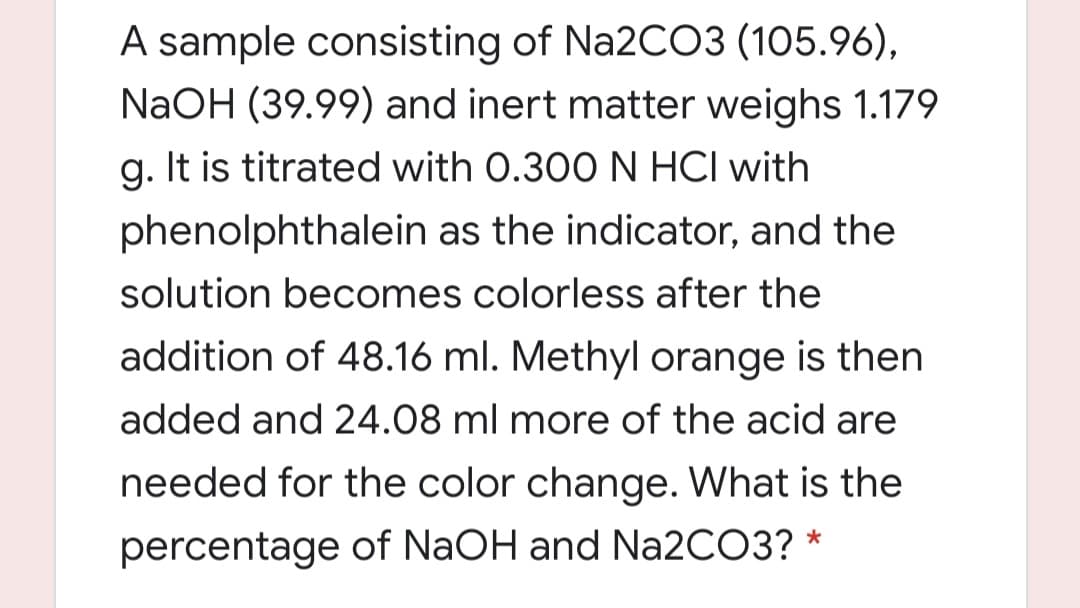
Transcribed Image Text:A sample consisting of Na2CO3 (105.96),
NaOH (39.99) and inert matter weighs 1.179
g. It is titrated with 0.300 N HCI with
phenolphthalein as the indicator, and the
solution becomes colorless after the
addition of 48.16 ml. Methyl orange is then
added and 24.08 ml more of the acid are
needed for the color change. What is the
percentage of NaOH and N22CO3? *
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

