A sample of limestone and other soil materials was heated, and the limestone decomposed to give calcium oxide and carbon dioxide. CACO3 (s) + CaO(s) + CO2 (g) A 2.085 g sample of limestone-containing material gave 0.831 g of CO2, in addition to CaO, after being heated at a high temperature. What was the mass percent of CaCO3 in the original sample? %
A sample of limestone and other soil materials was heated, and the limestone decomposed to give calcium oxide and carbon dioxide. CACO3 (s) + CaO(s) + CO2 (g) A 2.085 g sample of limestone-containing material gave 0.831 g of CO2, in addition to CaO, after being heated at a high temperature. What was the mass percent of CaCO3 in the original sample? %
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter3: Molecules, Moles, And Chemical Equations
Section: Chapter Questions
Problem 3.68PAE: 3.68 Magnesium is lighter than other structural metals, so it is increasingly important in the...
Related questions
Question
100%
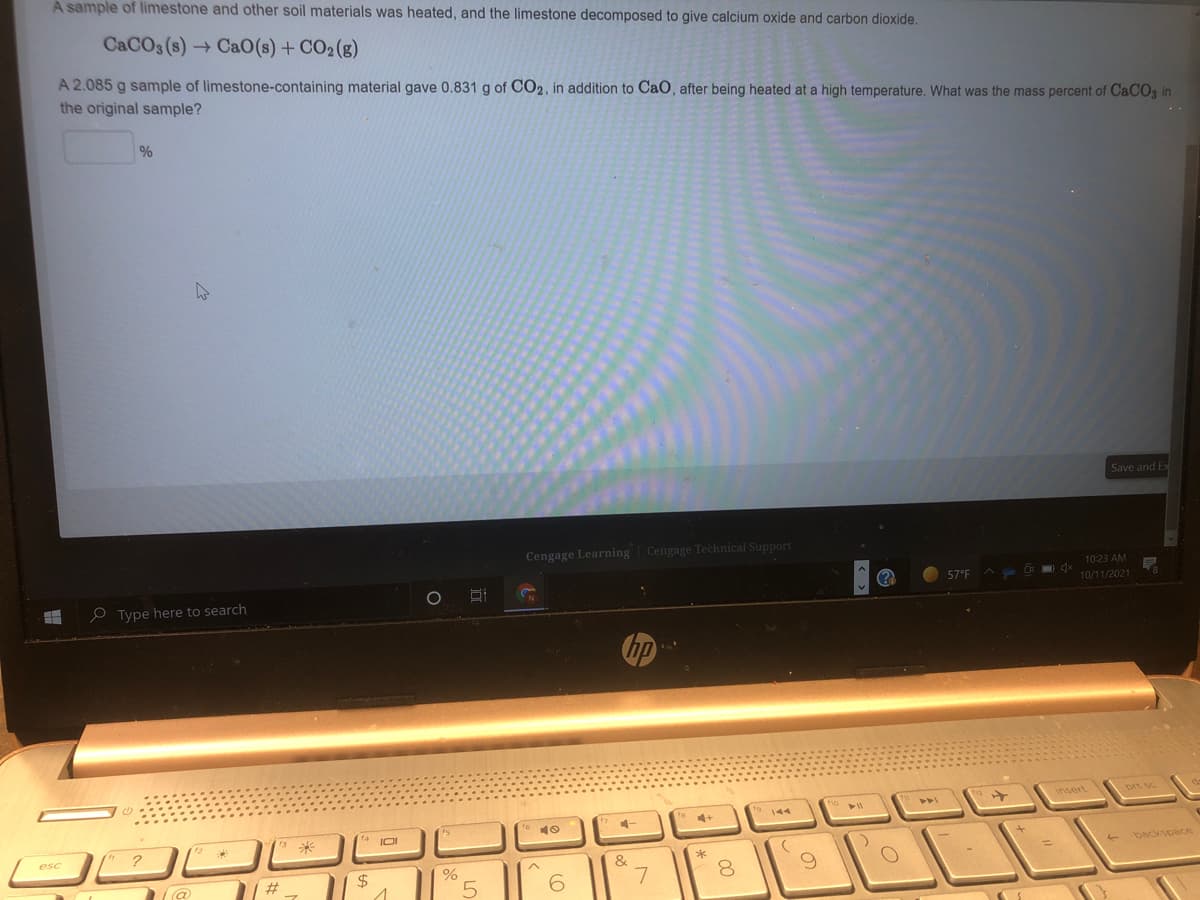
Transcribed Image Text:A sample of limestone and other soil materials was heated, and the limestone decomposed to give calcium oxide and carbon dioxide.
CACO3 (s) → CaO(s) + CO2(g)
A 2.085 g sample of limestone-containing material gave 0.831 g of CO2, in addition to CaO, after being heated at a high temperature. What was the mass percent of CACO3 in
the original sample?
%
Save and E
Cengage Learning Cengage Technical Support
10:23 AM
O Type here to search
(?
57°F
10/11/2021
insert
prt sc
4+
9144
4-
IOI
esc
*
backspace
&
#3
24
5.
7
00
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning